Acute Primary Angle Closure Glaucoma Prognostic Factors
Melanie Martel1, Nataliya Dorofeyev2, Sabrina Vangkhue2, Brandon Martel2, Matthew Martel3, Jordan P Hastings2 and Liliya Golas2*
1University of California Davis School of Medicine, USA
2California Northstate University College of Medicine, USA
3University of California, USA
Submission: September 14, 2021;Published: October 22, 2021
*Corresponding author: Liliya Golas, 11216 Trinity River Drive, California Northstate University College of Medicine, USA
How to cite this article: Melanie M, Nataliya D, Sabrina V, Brandon M, Matthew M, et al. Acute Primary Angle Closure Glaucoma Prognostic Factors. JOJ Ophthalmol. 2021; 8(5): 555750. DOI: 10.19080/JOJO.2021.08.555750
Abstract
Background: Acute primary angle closure glaucoma (APACG) is one of the most significant ocular emergencies presenting to emergency departments. This form of glaucoma is generally perceived as an acute ocular emergency which requires prompt ophthalmic referral for definitive treatment, which is almost universally surgical. At present however, prognostic factors on presentation are not well established and physicians do not have the ability to predict patient’s visual outcomes on presentation with reliable data.
Methods: In this retrospective case study, we analyze one hundred cases of acute primary angle closure glaucoma referred to our practice from four emergency departments (EDs) between 2008 and 2020 to statistically assess the characteristics, demographics, treatments, and clinical findings that affect outcomes.
Result & Conclusion: In our patient population emergent surgical intervention broke the acute attack in all patients, but only cured 27% of patients long term, leaving 73% of the patients requiring chronic treatment. This underscores the fact that acute angle closure glaucoma more often represents an exacerbation of a chronic disease requiring long-term treatment. We found that a presenting visual acuity (VA) of 20/200 or worse was the most significant prognostic factor in determining final VA. Other prognostic factors associated with poor visual outcomes included Black race, a need for continued intraocular pressure (IOP) lowering medications at one month of follow up, and a lack of health insurance. Asian race and a higher presenting IOP greater than 46 mmHg were suggestive of poor outcomes but were not statistically significant.
Keywords: Primary acute angle closure glaucoma; Emergency department; Eye; Vision; Treatment
Abbreviations: APACG: Acute Primary Angle Closure Glaucoma; ED: Emergency Department; VA: Visual Acuity; IOP: Intraocular Pressure; PACG: Primary Angle Closure Glaucoma; OD: Right Eye, OS: Left Eye; LPI: Laser Peripheral Iridotomy; NLP: No Light Perception
Introduction
Acute primary angle closure glaucoma (APACG) is one of the most important true ocular emergencies that a physician may encounter. Angle closure glaucoma results from a narrowing of the iridocorneal angle, causing obstruction of aqueous humor outflow and a rapidly elevated intraocular pressure. This process occurs in response to one of two mechanisms. In primary angle closure glaucoma (PACG), the flow of aqueous humor through the pupil is blocked. Conversely, secondary angle closure occurs when the iris is either pushed from behind or pulled anteriorly to narrow the angle. Both forms may present in an acute or chronic form. PACG tends to occur more acutely and patients with this etiology will frequently present to the emergency department (ED).
Patients with angle closure glaucoma often present to the emergency department complaining of severe ocular pain, headache, blurry vision, nausea, and vomiting, classically after transitioning from a light to dark environment. These symptoms are believed to be caused by a rapid increase in intraocular pressure (IOP). Nausea and vomiting generally occurs as a result of autonomic stimulation, while blurred vision and colored halos around lights are caused by corneal edema from the increased IOP pushing fluid into the cornea.
Physical exam may show a red eye with an elevated IOP (normal 10-20 mmHg), a fixed and mid-dilated pupil with poor reactivity to light, corneal edema, marked limbal conjunctival injection, and a shallow anterior chamber. Unfortunately, the gold standard for diagnosis involves evaluation of the anterior chamber angle with gonioscopy, [1,2] which is generally not available in an emergency department setting. However, assessing the anterior chamber can be done at bedside by shining a penlight perpendicularly to the direction of gaze and assessing for a shadow on the nasal iris. This test has been reported to have a sensitivity of 76% and a specificity of 81% [3].
There are several established risk factors for APACG including female sex, [4,5] Asian [6-9] or Inuit race, [10,11] older age [4,7] and certain drugs. [2] Anatomic predispositions include hyperopia, [12] a thick lens, [13] and an anatomically narrow anterior chamber. [12,14] Acute attacks have also been associated, albeit with less evidence, with emotional stress, [15,16] increased self-reported depression and anxiety, autonomic dysfunction, Valsalva maneuver, winter months, inflammation, [6] a larger iris area or thin iris [17].
In the emergency setting, patients presenting with APACG should be treated with IOP lowering agents, which may include pilocarpine, beta-blockers, alpha-agonists, carbonic anhydrase inhibitors, or hyperosmotic agents [1]. Urgent referral to an ophthalmologist should be made for definitive treatment, which is surgical in nature, and may include laser peripheral iridoplasty, [18,19] lens removal via phacoemulsification, [20-22] and/or peripheral iridotomy (PI) [23].
At present, there is no definitive data published to allow emergency department physicians to accurately determine or convey prognosis and likely necessary treatment modalities to their patients. Prognostic signs have been classically attributed to the duration of the attack and time to abate symptoms. [6,24] Peak IOP has been reported to not be predictive of long-term outcomes. [24-26] However, it has also been reported that increased IOP is associated with progression to chronic form,[6] requiring future lens extraction [27]. Additionally, the development of PACG after APACG in fellow eyes has been reported at 53%, even after prophylactic PI was completed. These authors reported that 21% of patients with an acute attack had progressive loss of visual fields on follow up, [28] with reports in populations of Asian individuals ranging from 33-72% [29].
With limited data on factors that affect long-term outcomes, our research aims to help provide prognostic clues into final visual acuity, the need for ocular surgery, and the likelihood of a patient requiring long-term treatment after the initial attack to control IOP. To our knowledge this is the first study to assess prognostic clues for long-term VA after APACG. This data can be used by the emergency department physician to help prognosticate the course of the disease and the expected outcomes.
Materials & Methods
We completed a retrospective case series of 100 patients referred for treatment of acute angle closure glaucoma from four local emergency departments for presumed APACG between 2008 and 2020. The study protocol was approved by the local Institutional Review Board of the California Northstate University, and the study was conducted in accordance with the Declaration of Helsinki. Data was obtained through retrospective chart review and included patient demographics, presenting symptoms, duration of symptoms, exam findings, initial and final VA, initial and final IOP, nature and duration of treatment and insurance status for measures of significance. T-tests and Pearson’s Chi squared tests were used to determine statistical significance for quantitative and categorical data respectively. A P-value of less than 0.05 was statistically significant.
Results
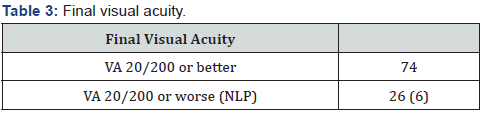
Patient age, sex, and ethnicity are presented in Table 1. Reported ethnicity breakdown was like the demographics of the surrounding area with a notable exception that the prevalence of Asian patients in our study was near double that of the community (25% vs. 14.3%). We also observed a lower-thanexpected number of Hispanic (12% vs. 21.6%) and Black patients (8% vs. 15.4%). The eyes were split at 47% right eye (OD) and 53% left eye (OS). Insured patients comprised 92%, and 8% were uninsured at the time of presentation. Average initial IOP was recorded at 44.8 mmHg (SD 11.7). All patients underwent a same day initial laser peripheral iridotomy (LPI) with a post-treatment average IOP of 18.6 (SD 10.6). A total of 31 patients required a repeated iridotomy for IOP control; patients requiring a second LPI had an average IOP of 29.1mmHg (SD 16.6) prior to the repeat procedure. Repeat LPI was completed 36 days after the initial intervention on average. Mean follow up time was 1.6 years with a maximum of 11.7 years.
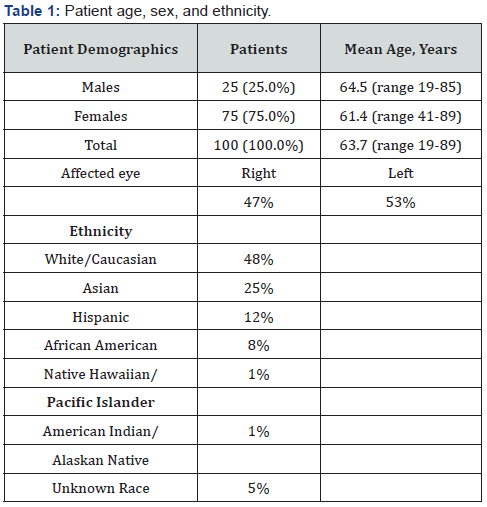
Presenting symptoms varied greatly. The most common presenting symptom was pain (70%). Additionally, 43% had blurry vision, 34% had headache, 34% complained of redness, and 25% experienced photophobia. Other less common symptoms included 20% with watering of the affected eye, 16% reported a sensation of pressure, 13% had burning, 13% had dryness, 11% complained of ocular discharge. 7% described their symptoms as an ocular discomfort, 6% had vomiting, 3% had swelling, 3% had double vision, 3% had a foreign body sensation, and 2% had dizziness (Table 2).
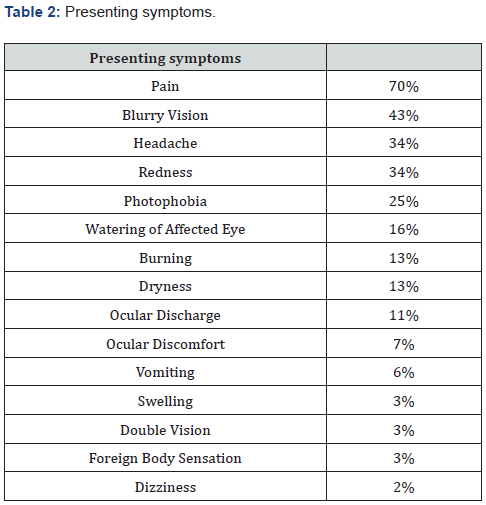
To determine the prognostic value of initial VA on presentation, we analyzed patients with a VA of better than 20/200 compared to those with VA of 20/200 or worse. Patients with an initial VA of 20/200 or worse were more likely to have a final VA of 20/200 or worse (44% vs. 2% p=6.9E-7), have a final VA of NLP (9% vs. 0%, p=0.042), and have higher initial IOP (48.2 +/- 12.0 vs. 40.5 +/-10.1, p=0.001). This occurred even though patients with an initial VA of 20/200 or worse were treated more aggressively with a greater number of medications (3.5 +/- 1.2 vs. 2.6 +/- 1.3 medications, p=0.002). To assess what factors were prognostic of blindness in the affected eye (defined as vision 20/200 or worse), we first stratified patients by their final visual outcome based on their most recent visit. The final VA on record showed 74 patients with a final VA of better than 20/200 and 26 patients with a final VA 20/200 or worse. Additionally, 6 of these patients had no light perception (NLP) (Table 3). Of the final VA patients with NLP, one of these six had a VA of NLP on presentation. Patients who ended up blind were more likely to seek medical attention sooner (2.3 +/-1.95 days vs. 4.2 +/- 6.19 days, p=0.044) and more likely to be on IOP medications long term (88% vs. 34%, p=0.016), defined as needing medications longer than one month after the initial attack.
Comparing patients with a final VA of NLP to those with final VA better than 20/200, NLP patients were significantly more likely to lack insurance (33% vs. 5%, p=0.012). Additionally, NLP patients had a higher, though not statistically significant, IOP following LPI (27.8 +/- 16.4 vs. 18.4 +/- 9.6 p=0.25). When analyzing final VA there was no significant difference in age, sex, need for a repeat PI, IOP after PI, number of medications used to treat the initial attack, or need for surgical lens removal between the groups. Presenting symptoms not specified above were not significantly different between groups and had no prognostic value. To address racial discrepancies and to better understand how race plays into prognosis we analyzed our data by patientreported race. Black patients were more likely to have final VA of 20/200 or worse when compared to White patients (p=0.02), while Asian patients were suggestive of similar outcomes relative to White patients (p=0.054). No other significant differences existed between racial groups including age, sex, insurance status, and need for long-term medication. Since length of symptoms is classically associated with prognosis, we looked at patients who presented with differing duration of symptoms. Surprisingly, there was no difference in age, sex, initial or final VA, insurance status, initial IOP, or need for cataract surgery when groups were split up by patients who presented within one day versus greater than one day and additionally when the groups were split at two or less days versus three or more days. This suggests that some patients clearly tolerated or experienced symptoms to different degrees.
Finally, since there have been discussions about the relevance of quantitative IOP level on presentation, we analyzed data based on the patient’s initial IOP on presentation. When broken up by initial IOP greater or less than or equal to 46 mm Hg, patients with higher IOP were more likely to have an initial VA of 20/200 or worse (70% vs. 40% p=0.003), but this was not significantly associated with final VA (30% vs. 19% p=0.19). With a higher cutoff value of 49 mmHg the association with final VA is slightly more predictive of final VA, but still not significant (p=0.11), indicating that initial IOP likely does not have prognostic value in determining final VA.
Discussion
In our study, 26% of patients ended up with final legal blindness, defined as a vision of 20/200 or worse and 6% with total loss of vision and no light perception. This is like other studies that report this data (range 5.6-24%) [26,30,31]. Additional studies report lower levels of blindness (6-11.4%) but use a higher cutoff value of blindness (20/400 or worse) [6,32]. Values from these studies with higher cutoffs are like our study’s incidence of a final visual acuity of NLP (6%). This grim prognosis of legal blindness in roughly one of four patients underscores the significance of the emergency department physician recognizing and properly managing this disease. Our results strongly suggest that initial VA 20/200 or worse can be used as a prognostic sign for final VA of 20/200 or worse. Additionally, Asian, or Black race, need for continued IOP lowering medications at one month follow up, and a presenting IOP of greater than 46 mmHg are all associated with eventual blindness in the affected eye. Unsurprisingly, a lack of insurance also correlated with worse visual outcomes, and this may be tied to a larger socioeconomic factor or access to care, which we were unable to analyze further with available data. To our surprise we were unable to find a correlation between duration of symptoms and final VA. This may be since there is a variation in the tolerance of symptoms early on by patients, subjective reporting, and the often subacute on chronic nature of the disease. Duration of the attack in APACG has previously been associated with poorer visual outcomes [33] and developing PACG [6,30]. However, this is not a definitive conclusion at present within the literature. In addition to our data, two other studies have failed to show a temporal correlation between time to treatment and final visual acuity. The first showed that there was minimal evidence that the duration of the attack influenced vision retained or recovered [34]. The second reported that a significant delay in treatment was not associated with poorer visual outcomes [35]. We agree with the conclusions that Hillman et al make in their paper; that is, that in addition to what we classically diagnose as APACG, there is likely a less devastating, subacute form of the disease, which can present similarly.
Evidence of a chronic process is supported by our data showing patients with a VA better than 20/200 presented after the onset of symptoms on average 1.9 days later than patients with an initial VA worse than 20/200. In addition, although numbers are too low to have high statistical power, of the six patients that presented after a week of symptoms, none had a final VA 20/200 or worse, but 83% required medication long term. This suggests that these patients may have a presentation more consistent with a subacute on chronic process. It should also be noted that although we did not find a statistically significant difference in time to presentation, we believe there is still benefit to early diagnosis and treatment. This is evidenced by the fact that of those patients who were free of symptoms and medications long term (i.e., those who truly were cured by LPI shortly after presentation), 67% of them presented within two days, and 80% presented within four days. As these cases do have better outcomes with a quicker time to LPI, we believe that early treatment to lower IOP does maximize the chance of a cure and is still the standard of care and treatment for these cases should not be delayed.
We recognize that there are limitations in our study, mainly due to its retrospective nature. Additionally, the subjective nature of reporting of symptoms and their duration may have introduces bias. Finally, small sample sizes in specific ethnic patient groups and in NLP patients may make specific conclusions about these groups less reliable.
Conclusion
Our results demonstrate that the strongest predictive factor for poor final visual outcome is a presenting VA of 20/200 or worse. Additionally, our work supports previous data suggesting that IOP on presentation is unrelated to final visual outcomes [24]. This may be since the mean ocular perfusion pressure is still typically higher than the average presenting IOP as observed in our patient population [36,37]. However, there still may be some prognostic value in the initial IOP, with higher values correlating, albeit insignificantly, with poorer final VA. Finally, we recommend that APACG should be approached as an acute presentation of a chronic disease, which requires long-term treatment and follow up after the initial frequently surgical intervention.
References
- Weinreb RN, Aung T, Medeiros FA (2014) The pathophysiology and treatment of glaucoma: A review. Journal of the American Medical Association.
- Yang MC, Lin KY (2019) “Drug-induced acute angle-closure glaucoma: A review. Journal of Current Glaucoma Practice. 13(3): 104-109.
- Mingguang He, Wenyong Huang, David S Friedman, Changfan Wu, Yingfeng Zheng, et al. (2007) Slit lamp-simulated oblique flashlight test in the detection of narrow angles in Chinese eyes: The Liwan eye study. Investig Ophthalmol Vis Sci 48: 5459-5463.
- Seah SK, Foster PJ, Chew PT, Jap A, Oen F, et al. (1997) Incidence of acute primary angle-closure glaucoma in singapore: An island-wide survey. Arch Ophthalmol 115(11): 1436-1440.
- Quigley H, Broman AT (2006) The number of people with glaucoma worldwide in 2010 and 2020. British Journal of Ophthalmology 90(3): 262-267.
- Andreatta W, Elaroud I, Nightingale P, Nessim M (2015) Long-term outcomes after acute primary angle closure in a White Caucasian population. BMC Ophthalmol 15: 108.
- Foster PJ, Baasanhu J, Alsbirk PH, Munkhbayar D, Uranchimeg D, et al. (1996) Glaucoma in Mongolia: A population-based survey in Hovsgol province, northern Mongolia. Arch Ophthalmol 84(2): 186-192.
- Nguyen N, Mora JS, Gaffney MM, Ma AS, Wong PC, et al. (1996) A high prevalence of occludable angles in a Vietnamese population. Ophthalmology S0161-6420(96): 30488.
- Lai JS, Liu DT, Tham CC, Li RT, Lam DS (2001) Epidemiology of acute primary angle-closure glaucoma in the Hong Kong Chinese population: prospective study. Hong Kong Med J 7(2):118-123.
- Bourne RRA, Sørensen KE, Klauber A, Foster PJ, Johnson GJ, et al. (2001) Glaucoma in East Greenlandic Inuit - A population survey in Ittoqqortoormiit (Scoresbysund). Acta Ophthalmol Scand 79(5): 462-467
- Arkell SM, Korshin OM, Sommer A, Taylor HR, Tielsch JM (1987) The Prevalence of Glaucoma Among Eskimos of Northwest Alaska. Arch Ophthalmol 105(4): 482-485.
- Van Herick W, Shaffer RN, Schwartz A (1969) Estimation of width of angle of anterior chamber. Incidence and significance of the narrow angle. Am J Ophthalmol 68(4): 626-629.
- Lowe RF (1970) Aetiology of the anatomical basis for primary angle-closure glaucoma. Biometrical comparisons between normal eyes and eyes with primary angle-closure glaucoma. Br J Ophthalmol 54(3): 161-169.
- Alsbirk FH (1992) Anatomical risk factors in primary angle-closure glaucoma - A ten year follow up survey based on limbal and axial anterior chamber depths in a high-risk population. Int Ophthalmol 16(4-5): 265-272.
- Talluto D, Feitl M, Allee S (1998) Simultaneous angle closure in twins. J Glaucoma 7(1):68-69.
- Cohen SI, HJ (1972) Life events and the onset of acute closed-angle glaucoma. J Psychosom Res 3999(72): 90086-90094.
- Lifang Liu, Xinyu Liu, Chukai Huang, Geng Wang, Di Ma, et al. (2017) Associated factors of acute primary angle closure glaucoma in a sub-group of Chinese people: Comparison between attack eyes and normal controls. Sci Rep 7(14885).
- Shida Chen, Jianhua Lv, Sujie Fan, Hong Zhang, Lin Xie, et al. (2017) Laser peripheral iridotomy versus laser peripheral iridotomy plus laser peripheral iridoplasty in the treatment of multi-mechanism angle closure: Study protocol for a randomized controlled trial. Trials 18(1): 130.
- Lam DSC, Lai JSM, Tham CCY, Chua JKH, Poon ASY (2002) Argon laser peripheral iridoplasty versus conventional systemic medical therapy in treatment of acute primary angle-closure glaucoma: A prospective, randomized, controlled trial. Ophthalmology 109(9): 1591-1596.
- Hellen CS Römkens, Henny JM Beckers, Jan SAG Schouten, Rudy MMA Nuijts, Tos TJM Berendschot, et al. (2018) Early Phacoemulsification after Acute Angle Closure in Patients with Coexisting Cataract. J Glaucoma 27(8): 711-716.
- Dennis SC Lam, Dexter YL Leung, Clement CY Tham, Felix CH Li, Yolanda YY Kwong, et al. (2008) Randomized Trial of Early Phacoemulsification versus Peripheral Iridotomy to Prevent Intraocular Pressure Rise after Acute Primary Angle Closure. Ophthalmology 115(7): 1134-1140.
- Yun-Hsuan Lin, Cheng-Hsiu Wu, Shih-Ming Huang, Chen Hsieh, Henry Shen-Lih Chen, et al. (2020) Early versus Delayed Phacoemulsification and Intraocular Lens Implantation for Acute Primary Angle-Closure. J Ophthalmol 2020(8319570).
- Chan PP, Pang JC, Tham CC (2019) Acute primary angle closure–treatment strategies, evidence and economic considerations. Eye (Basingstoke) 33(1): 110-119.
- David R, Tessler Z, Yassur Y (1985) Long-term outcome of primary acute angle-closure glaucoma. Br J Ophthalmol 15:108.
- Playfair TJ, Watson PG (1979) Management of chronic or intermittent primary angle-closure glaucoma: A long-term follow-up of the results of peripheral iridectomy used as an initial procedure. Br J Ophthalmol 63(1): 23-28.
- Tin Aung, David S Friedman, Paul T K Chew, Leonard P Ang, Gus Gazzard, et al. (2004) Long-term outcomes in asians after acute primary angle closure. Ophthalmology 111(8):1464-1469.
- Bo J, Changulani T, Cheng ML, Tatham AJ (2018) Outcome Following Laser Peripheral Iridotomy and Predictors of Future Lens Extraction. J Glaucoma 27(3): 275-280.
- Fea AM, Dallorto L, Lavia C, Pignata G, Rolle T, Aung T (2017) Long-term outcomes after acute primary angle closure of Caucasian chronic angle closure glaucoma patients. Clin Exp Ophthalmol 46(3): 232-239.
- Ang LPS, Ang LPK (2008) Current understanding of the treatment and outcome of acute primary angle-closure glaucoma: An asian perspective. Annals of the Academy of Medicine Singapore 37(3): 210-215.
- Tan AM, Loon SC, Chew PTK (2009) Outcomes following acute primary angle closure in an Asian population. Clin Exp Ophthalmol 37(5): 467-472.
- Lowe RF (1973) Primary angle-closure glaucoma: A review 5 years after bilateral surgery. Br J Ophthalmol 57(7): 457-463.
- Lee JWY, Wong BKT, Yick DWF, Wong IYH, Yuen CYF, et al. (2014) Primary acute angle closure: Long-term clinical outcomes over a 10-year period in the Chinese population. Int Ophthalmol 34(2):165-169.
- Buckley SA, Reeves B, Burdon M, Moorman C, Wheatcroft S, et al. (1994) Acute angle closure glaucoma: Relative failure of YAG iridotomy in affected eyes and factors influencing outcome. Br J Ophthalmol 78(7): 529-533.
- Ingram RM, Ennis JR (1983) Acute glaucoma: Results of treatment by bilateral simultaneous iridectomy, now without admission to hospital. Br J Ophthalmol 67(6): 367-371.
- Hillman JS (1979) Acute closed-angle glaucoma: An investigation into the effect of delay in treatment. Br J Ophthalmol 63(12): 817-821.
- Memarzadeh F, Ying-Lai M, Chung J, Azen SP, Varma R (2010) Blood pressure, perfusion pressure, and open-angle glaucoma: The Los Angeles Latino eye study. Investig Ophthalmol Vis Sci 51(6): 2813-3326.
- Zheng Y, Wong TY, Mitchell P, Friedman DS, He M, et al. (2010) Distribution of ocular perfusion pressure and its relationship with open-angle glaucoma: The singapore malay eye study. Investig Ophthalmol Vis Sci 51(7): 3327-3839.






























