Treating Macular Edema with Nepafenac in Neuroretinitis
Paz T, Rappaport D, Leiba H, Goldfether S and Levy N*
Ophthalmology Department, Kaplan Medical Center, Israel
Submission: October 02, 2017; Published: November 15, 2017
*Corresponding author: Levy Niv, Ophthalmology Department, Kaplan Medical Center, 1 Pasternak Road, Rehovot 76100, Israel; Tel: +972-54-5967283; Email: Niv.levi@mail.hujiac.il
How to cite this article: Paz T, Rappaport D, Leiba H, Goldfether S and Levy N*. Treating Macular Edema with Nepafenac in Neuroretinitis. JOJ Ophthal. 2017; 5(4): 555666. DOI: 10.19080/JOJO.2017.05.555666.
Abstract
Purpose: Report a case of neuroretinitis and macular edema partially responsive to systemic corticosteroids, who was successfully treated with Nepafenac 0.1%.
Observations: A 46-year-old woman, presented with decreased vision in her left eye for the past 5 days. Visual acuity (VA) on presentation was 6/6 in her right eye and FC in her left eye, with negative afferent pupillary defect. Fundus examination in the left eye revealed swelling and elevation of the optic disk and macular edema with macular star exudates. Macular Optical Coherent Tomography (OCT) showed presence of intraretinal and subretinal fluid. The patient was started on IV solumedrol 1 gram per day and oral azithromycin 500 mg daily, and was released a week later with oral prednisone, with VA of 6/15 in the left eye. Due to an increase in the subretinal fluid under tapering down of the steroids, an attempt to raise the dosage was made with partial decrease of the subretinal fluid, but with re-accumulation of the fluid with a second attempt of tapering down. Steroids were discontinued and treatment with nepafenac 0.1% was initiated three times daily (TID). One month after TID administration of nepafenac 0.1% alone, retinal thickness decreased and visual acuity improved to 6/6, with normal disc appearance and residual macular exudates. Macular OCT revealed complete resolution of the residual subretinal fluid. In light of the improvement in visual acuity and absorption of the subretinal fluid under nepafenac treatment, the patient continued treatment with nepafenac for another 2 months and remained well on follow-up.
Conclusions: Nepafenac 0.1% may be beneficial and hastens the visual recovery of patients with CME due to neuroretinitis. Further studies are needed to investigate the role of topical nepafenac, which may be an alternative to systemic corticosteroids in treating neuroretinitis presenting with macular edema.
Keywords: Borage, Retinal venous pressure, Ophthalmodynamometry, Flammer syndrome
Introduction
Neuroretinitis is an inflammatory disorder characterized by optic disc edema and subsequent formation of a macular star figure [1]. The current understanding of neuroretinitis is that there is presence of inflammation, which leads to an increased permeability of optic disc vasculature with secondary leakage into surrounding retina [1,2]. The resorption of the accumulated fluid in the outer plexiform layers leaves behind lipid precipitates in a stellate pattern around the macula, forming a macular star [3].
Cystoid macular edema (CME) is the cystic accumulation of fluid in the central retina [4]. Prolonged accumulation of intracellular fluid in macular edema can cause permanent and lasting damage to the retina [4]. CME can be a serious consequence of numerous ocular procedures and conditions, including cataract surgery, ocular inflammatory disease, retinal vascular diseases, and tractional disorders [5].The incidence of subclinical angiographic CME is approximately 20%-30% of uncomplicated cataract surgery cases, while acute clinically significant CME has been reported from 1%-2% of patients following uncomplicated phacoemulsification. Macular edema secondary to uveitis is also a common problem, occurring in up to 48% of uveitic eyes [6]. CME is not a disease itself, rather the endpoint of a variety of processes that lead to the accumulation of fluid in the central retina [5]. Macular edema may develop in a variety of conditions that cause hyper-permeability of capillaries in the optic nerve head. Protein and lipid-rich exudative fluid may extend from the optic nerve into the macular region, in some cases leading to a stellate pattern of yellow macular exudate as the serous fluid component resolves. Conditions that may cause this clinical picture include hypertensive papillopathy, diabetic papillopathy, idiopathic and infectious neuroretinitis, and other inflammatory causes of optic disc swelling such as sarcoidosis [2].
The current consensus is that CME is at least partly mediated by inflammatory processes, with increased prostaglandin- mediated vascular permeability [4]. Injury and disease may stimulate the release of prostaglandins. Prostaglandins released locally into the tissues lead to a breakdown of the blood- aqueous or blood-retina barriers, followed by vascular leakage of serum into the retinal interstitium [4]. If prostaglandins are a major contributor to the development of CME, inhibition of prostaglandin synthesis with nonsteroidal anti-inflammatory drugs (NSAIDs) seems a reasonable approach for CME treatment.
Case Report
A 46-year-old Caucasian woman, presented with decreased vision in her left eye for the past 5 days, accompanied with tension headache. She had no fever and fatigue, and denied weight loss. Her past medical and family histories were unremarkable.
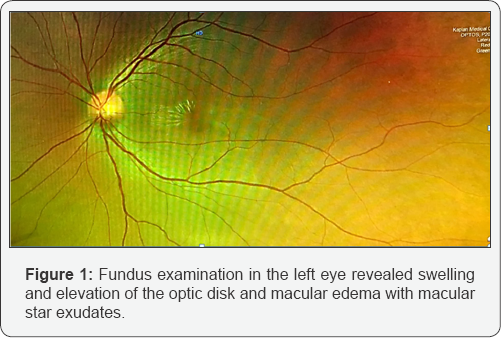
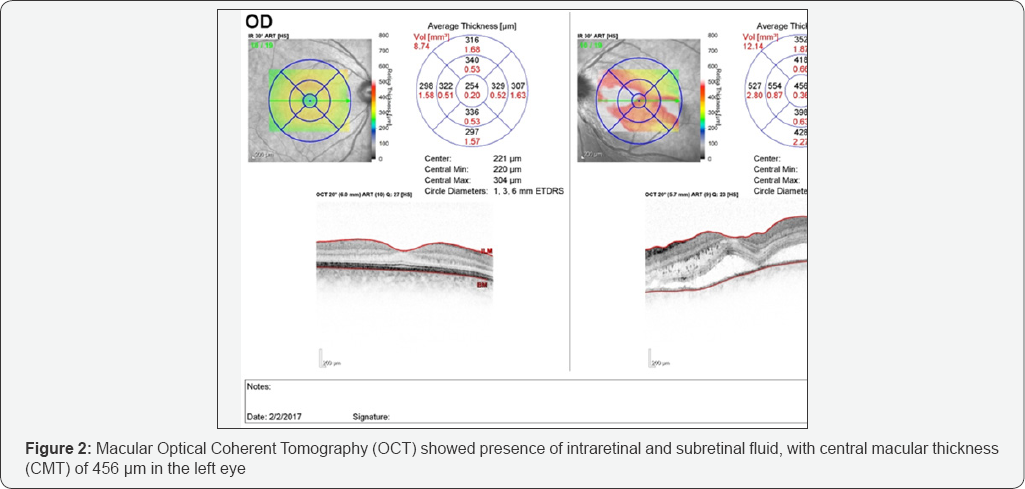
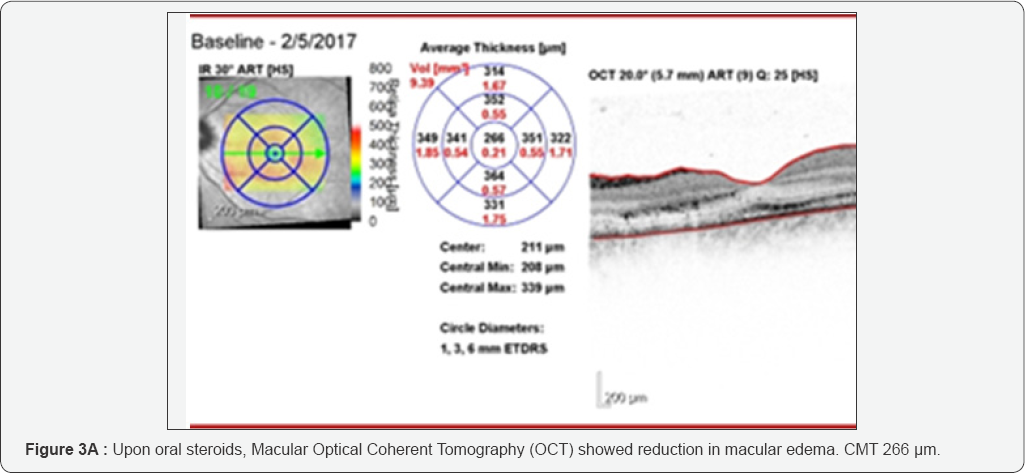
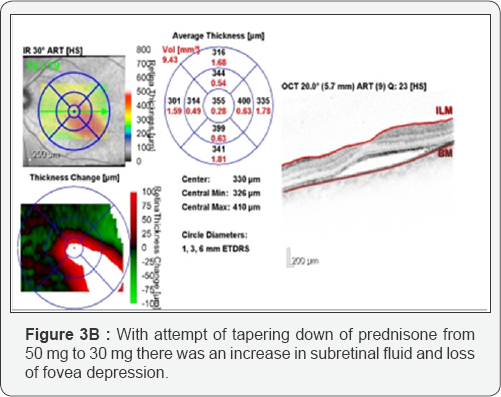
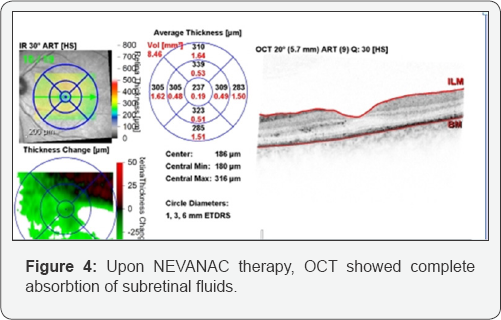
On examination, the patient's visual acuity (VA) was 6/6 OD and FC OS, with negative afferent pupillary defect. Fundus examination in her left eye revealed swelling and elevation of the optic disk and macular edema with macular star exudates (Figure 1). Macular Optical Coherent Tomography (OCT) showed presence of intraretinal and subretinal fluid, with central macular thickness (CMT) of 456 pm in the left eye (Figure 2). The patient was hospitalized with suspected neuroretinitis. Laboratory findings were unremarkable, including serologic test for bartonella henselae and blood test for AQP-4 antibodies. MRI of the brain and orbits showed hyper-intense periventricular lesions in the white matter, without ocular findings. CXR showed no sign of pleural effusion or infiltrate. Lumbar puncture was performed with normal protein and white blood cell counts. PCR was sent for CMV and HSV with negative results, and a test from the CSF was sent for detection of oligoclonal bands, also with negative results. The patient was started on IV solumedrol 1 gram per day and oral azithromycin 500 mg daily, and was released a week later, upon oral prednisone 50 mg, with VA of 6/15 in the left eye. Due to an increase in the subretinal fluid under tapering down of the steroids, an attempt to raise the dosage was made with partial decrease of the subretinal fluid, but with re-accumulation of the fluid with a second attempt of tapering down (Figure 3A & 3B). Steroids were discontinued and treatment with nepafenac 0.1% was initiated three times daily (TID). One month after TID administration of nepafenac 0.1% alone, retinal thickness had improved to 237pm and visual acuity had improved to 6/6, with normal disc appearance and residual macular exudates. Macular OCT revealed complete resolution of the residual subretinal fluid (Figure 4). In light of the improvement in visual acuity and absorption of the subretinal fluid under nepafenac treatment, the patient continued treatment with Nepafenac for another 2 months and remained well on follow-up. Our patient completed 12 weeks of topical nepafenac, and the treatment led to improvement in visual acuity and retinal thickness, with complete resolution of the residual subretinal fluid, successfully treating macular edema.
Discussion
Many studies have shown an association between CME and inflammation [5,6]. For this reason, CME is commonly treated with anti-inflammatory agents. Corticosteroids can effectively prevent and treat CME but are associated with serious side effects, including elevated intraocular pressure (IOP), posterior subcapsular cataract, and increased susceptibility to concomitant infections [6]. Thus, safer alternative treatments are desirable. Topical NSAIDs have demonstrated efficacy in prophylaxis and/ or treatment of CME [4,6,7]. Because corticosteroids and NSAIDs inhibit prostaglandin synthesis by different mechanisms, an additive or synergistic effect is possible with combined therapy. Studies have confirmed the benefits of combination therapy [7], and it is now widely accepted that a combination of topical NSAID and corticosteroids be initiated upon documentation of clinical CME [6]. The beneficial effects of NSAIDs over corticosteroids include stabilization of intraocular pressure, provision of analgesia and reduction of the risk of secondary infections [5]. Prospective studies have proven the beneficial effect of combination NSAID-corticosteroid therapy in preventing CME compared to corticosteroid therapy alone [4,7].
NSAIDs may have a modulating effect on ocular inflammation and pain through the prevention of prostaglandin synthesis via cyclooxygenase inhibition [8]. In addition, for intraocular surgery, NSAIDs can help to prevent intraoperative miosis, reduce ocular pain, decrease postoperative inflammation and prevent cystoid macular edema [9]. Newer-generation NSAIDs have emerged in recent years for the treatment of ocular pain and inflammation. Newer NSAIDs, including bromfenac and nepafenac, were formulated to have great-er corneal penetration and, in preclinical studies, were shown to have higher concentrations in ocular tissues, including the retina [8,9]. Given the potential for better drug delivery to the posterior segment with newer NSAIDs and a growing body of evidence that implicates inflammation in the pathogenesis of macular edema associated with multiple etiologies, there is renewed in-terest in the role of topical NSAIDs to treat retinal disease.
Nepafenac 0.1% is a novel NSAID that is currently approved for the treatment of pain or inflammation associated with cataract surgery [10], and is the only ocular NSAID with a prodrug structure [8]. Preclinical data suggest that nepafenac's pharmacokinetic properties should provide optimal antiinflammatory effect to the key target tissues in the posterior segment. The data suggest that nepafenac may have greater antiinflammatory efficacy in the uvea and retina/choroid, and may suppress prostaglandins synthesis longer than conventional topical ocular NSAIDs [4]. Retinal, choroidal and vitreous diseases may be the target of future nepafenac studies, either as monotherapy or as combination treatments [10]. Intraocular hydrolases convert nepafenac to the more active metabolite amfenac [8]. These hydrolases are present in ocular tissues, such as the cornea and iris/ciliary body, but are at their highest concentrations in the retina/choroid [8]. As a result, the more active form of the drug, amfenac, is effectively targeted to the inflamed macula in cases of CME [11]. Although The efficacy of topical nepafenac 0.1% for inflammation in the anterior segment and pain control after cataract surgery is well established [4,12], the proposed clinical efficacy for alleviating posterior segment inflammation and CME due to the pharmacodynamic differences of this drug compared with other NSAIDs requires further study.
Topically administered nepafenac 0.1% may be more effective than other NSAIDs in preventing and treating posterior segment inflammation [4]. First, nepafenac exhibits enhanced corneal permeability. The rate of corneal permeation of nepafenac is approximately fourfold faster than that of diclofenac [4,13], allowing for greater intraocular drug accumulation [13]. The rate of hydrolysis of nepafenac to the more active amfenac is much greater in the retina/choroid (approximately 20 times) than in the iris/ciliary body [8,13]. This increased absorption and intraocular activation should improve efficacy in the posterior segment and minimize corneal irritation. Ex vivo studies using rabbit retina/choroid tissues demonstrated that nepafenac 0.1% reached its peak activity (suppression of prostaglandin production) sooner than diclofenac 0.1% (40 minutes vs 80 minutes) [8]. The administration of nepafenac also produces rapid and long lasting inhibition of prostaglandin synthesis relative to a conventional NSAID (6 h vs. 20 min), thereby stabilizing the blood-retina barrier [8]. Prostaglandin synthesis is inhibited for more than 6 hours in the iris/ciliary body and for up to at least 4 hours in the retina/choroid, based on ex vivo studies [4,8]. Finally, results from an in vivo rabbit study of concanavalin A-induced retinal edema showed that nepafenac inhibited retinal inflammation, as measured by blood-retinal barrier breakdown and prostaglandin synthesis, significantly better than either diclofenac or ketorolac [14].
Although no randomized, controlled clinical trials have yet been reported on the ability of nepafenac 0.1% to treat CME, evidence is mounting that this drug has promising activity against macular edema of varying etiologies. One reported case series illustrates the positive impact of nepafenac on acute and chronic CME, even in patients who have failed combination treatment with a steroid and a conventional NSAID [11]. Another study concluded that topical nepafenac is effective in patients with chronic pseudophakic CME unresponsive to other topical medications [15]. A similar study demonstrated that topical nepafenac, with or without concomitant steroids, reduced retinal thickness and improved visual acuity in patients with acute pseudophakic, chronic pseudophakic, and recalcitrant uveitic CME [16]. Another report focuses on the successful use of nepafenac for the treatment of chronic uveitic CME [17]. Other reports demonstrate the resolution of macular edema by nepafenae 0.1% in steroid responders [18], and in patients with diabetic macular edema [19]. In addition to CME treatment, nepafenac 0.1% may also have prophylactic activity against CME, as suggested by a retrospective study of 450 consecutive patients with CME showing that those treated with prednisolone alone had a higher incidence of CME than those treated with prednisolone and nepafenac 0.1% [20].
In our case report, topical nepafenac 0.1% was started with the aim of treating the component of inflammatory macular edema, after a failure to reach resolution of residual subretinal fluid with tapering down of steroids. The patient demonstrated good early visual recovery from FC to 6/6 in 4weeks, which justified the continued usage of nepafenac. The early improvement was evidenced by OCT, as shown in Figure 4, where there is reduction of intraretinal and subretinal fluid as compared to Figure 3B. This suggests that topical nepafenac 0.1% used in conjunction with oral antibiotics in a patient with neuroretinitis is safe and beneficial, as shown in another case report [3].
The positive outcomes of the patient presented here, showing resolution of macular edema and improvement in visual acuity, provide additional evidence that nepafenac can effectively treat multiple types of CME. Therefore, this case report adds to the body of evidence supporting an important role for nepafenac 0.1% in the treatment of macular edema. Although the macular edema improved quickly in this case, it must be noted that acute CME typically resolves spontaneously, making it difficult to determine the role of nepafenac on this improvement.
The enhanced permeability, targeted bioactivation, and greater duration of action of nepafenac may lead to improved efficacy in the posterior segment over other NSAIDs lacking these properties. Nepafenac's anti-inflammatory properties and its pharmacodynamics support a prospective role for nepafenac in conditions that are caused by prostaglandin -mediated vascular leakage, such as anterior chamber inflammation and cystoid macular edema following cataract surgery, and in the prevention of CME. This makes nepafenac a unique agent that targets control of both anterior and posterior segment inflammation.
Conclusion
Ophthalmologists have been evalu-ating the role of NSAIDs in the treatment of retinal disease for years, and future well- designed trials are needed to better define their utility in the treatment and prevention of macular edema, whether alone or in combination with other topical and/or in-travitreal therapeutics. The case report presented here adds to the growing body of knowledge that the new generation NSAIDs may be useful to treat CME as primary therapy. Further studies of nepafenac in placebo-controlled trials are warranted to investigate the role of topical nepafenac as an alternative to systemic corticosteroids in cases of macular edema in patients with neuroretinitis.
Conflict of Interest
The authors declare that there is no conflict of interests regarding the publication of this paper.
Patient Consent
This report does not contain any personal information that could lead to the identification of the patient.
References
- Purvin V, Sundaram S, Kawasaki A (2011) Neuroretinitis: review of the literature and new observations. J Neuroophthalmol 31(1): 58-68.
- Johnson MW (2009) Etiology and treatment of macular edema. Am J Ophthalmol 147(1): 11-21.
- Chee KW, Shirlyna N (2016) Topical NSAID an option for early resolution of macular edema in neuroretinitis?: A case report. Aperito J Ophthalmol 2: 2.
- Lindstrom R, Kim T (2006) Ocular permeation and inhibition of retinal inflammation: an examination of data and expert opinion on the clinical utility of nepafenac. Curr Med Res Opin 22(2): 397-404.
- Cho H, Madu A (2009) Etiology and treatment of the inflammatory causes of cystoid macular edema. J Inflamm Res 2: 37-43.
- Hariprasad SM, Akduman L (2009) Treatment of cystoid macular edema with the new-generation NSAID nepafenac 0.1%. Clin Ophthalmol 3: 147-154.
- Heier JS, Topping TM, Baumann W, Dirks MS, Chern S (2000) Ketorolac versus prednisolone versus combination therapy in the treatment of acute pseudophakic cystoid macular edema. Ophthalmology 107(11): 2034-2038.
- Gamache DA, Graff G, Brady MT, Spellman JM, Yanni JM (2000) Nepafenac, a unique nonsteroidal prodrug with potential utility in the treatment of trauma-induced ocular inflammation: I. Assessment of anti-inflammatory efficacy. Inflammation 24(4): 357-370.
- Kim SJ, Flach AJ, Jampol LM (2010) Nonsteroidal anti-inflammatory drugs in ophthalmology. Surv Ophthalmol 55(2): 108-133.
- Rodrigues EB, Farah ME (2016) Nonsteroidal Anti-Inflammatory Drugs in the Treatment of Retinal Diseases. Dev Ophthalmol 55: 212-220.
- Hariprasad SM, Callanan D (2007) Cystoid and diabetic macular edema treated with Nepafenac 0.1%. J Ocul Pharmacol Ther 23(6): 585-590.
- Lane SS, Modi SS, Lehmann RP, Holland EJ (2007) Nepafenac ophthalmic suspension 0.1% for the prevention and treatment of ocular inflammation associated with cataract surgery. J Cataract Refract Surg 33(1): 53-58.
- Ke TL, Graff G, Spellman JM, Yanni JM (2000) Nepafenac, a unique nonsteroidal prodrug with potential utility in the treatment of trauma- induced ocular inflammation: II. In vitro bioactivation and permeation of external ocular barriers. Inflammation 24(4): 371-384.
- Kapin MA, Yanni JM, Brady MT, McDonough TJ, Flanagan JG, et al. (2000) Inflammation-mediated retinal edema in the rabbit is inhibited by topical nepafenac. Inflammation 27(5): 281-291.
- Akduman L, Clever JA, Feman S (2007) Nepafenac used for the treatment chronic pseudophakic cystoid macular edema. The Retina Society, Boston, MA, USA.
- Hariprasad SM, Mieler WF (2007) Treatment of acute pseudophakic, chronic pseudophakic, and recalcitrant uveitic cystoid macular edema with nepafenac 0.1% (Nevanac). The Retina Society, Boston, MA, USA.
- Hariprasad SM, Callanan D (2008) Topical nepafenac 0.1% for treatment of chronic uveitic cystoid macular edema. Retin Cases Brief Rep 2(4): 304-308.
- Warren KA, Fox JE (2008) Topical nepafenac as an alternate treatment for cystoid macular edema in steroid responsive patients. Retina 28(10): 1427-1434.
- Callanan D, Williams P (2008) Topical nepafenac in the treatment of diabetic macular edema. Clin Opthalmol 2(4): 999-1002.
- Wolf EJ, Braunstein A, Shih C, Braunstein RE (2007) Incidence of visually significant pseudophakic macular edema after uneventful phacoemulsification in patients treated with nepafenac. J Cataract Refract Surg 33(9): 1546-1549.






























