Aldose Reductase Inhibitors from Nature: A New Hope for Treatment of Cataract
Farid A Badria1*, Diaaeldin M Elimam1, Mohammed SI Elabshihy2 and Ahmed S Ibrahim3
1Department of Pharmacognosy, Mansoura University, Egypt
2Department of Ophthalmology, AlAzhar Universitsy, Egypt
3Departments of Biochemistry, Mansoura University, Egypt
Submission: August 08, 2017; Published: September 22, 2017
*Corresponding author: Farid A Badria, Department of Pharmacognosy, Faculty of Pharmacy, Mansoura University, Egypt, Email: faridbadria@gmail.com
How to cite this article: Farid A B, Diaaeldin M E, Mohammed S E, Ahmed S I. Aldose Reductase Inhibitors from Nature: A New Hope for Treatment of Cataract. JOJ Ophthal. 2017; 5(1): 555655. DOI: 10.19080/JOJO.2017.05.555655.
Introduction
Diabetes mellitus is collection of metabolic disorders characterized by high serum glucose level in the body that is due to low insulin secretion or due to cellular unresponsiveness toward produced insulin. This high level of glucose produces the conventional symptoms of polyphagia, polydipsia and polyuria [1] . Uncontrolled diabetes can cause diverse complications, diabetic ketoacidosis and non ketotic hyperosmolar coma fall under acute complications [2] while chronic complications comprise multiple tissue damage that results in stroke, cardiovascular disease, foot ulcers, renal failure and eye damage [3].
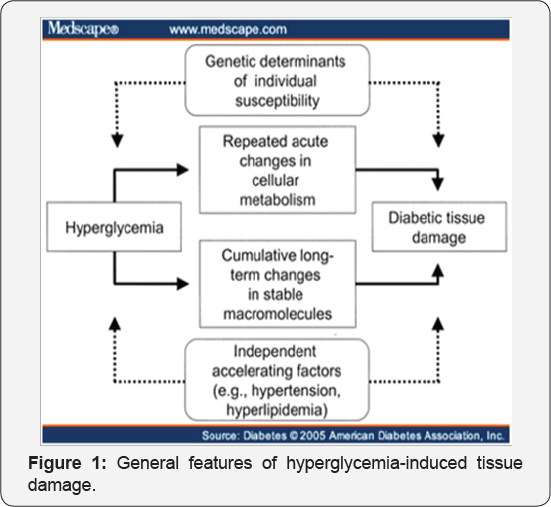
The general features of hyperglycemia-induced tissue damage are shown schematically in (Figure 1). The DCCT (Diabetes Control and Complications Trial) and the UKPDS (U.K.Prospective Diabetes Study) established that hyperglycemia (far left of the figure) causes the clinically manifest diabetic tissue damage (far right). It is affected by genetic determinants of individual susceptibility (top box) and by independent accelerating factors such as hypertension (bottom box), the (inner boxes) is the mechanisms that mediate the tissue- damaging effects of hyperglycemia [4].
Tissue-damaging effects of hyperglycemia mean damage to a specific subset of cell types, mesangial cells (renal glomerulus), capillary endothelial cells (retina) and neurons and Schwann cells (peripheral nerves). These cells are especially vulnerable to high glucose (hyperglycemia baths all the cells of every tissue) due to that they cannot reduce the transport of glucose inside them under hyperglycemia (other cells are able to do so), so their internal glucose concentration elevate. This is significant, because it indicate involving mechanisms going on inside these cells, rather than outside [5,6]. In the tissue damaging mechanisms polyol pathway was the first discovered mechanism[7] . Then, in the late 1970's, a second mechanism emerged, increased formation of Advanced Glycation End products (AGEs)[8] . In the late 1980's and early 1990’s, a third mechanism was elucidated, hyperglycemia-induced activation of protein kinase C (PKC) isoforms [9]. And finally in the late 1990's, latest mechanism was clarified, increased hexosamine pathway flux and consequent over-modification of proteins by N-acetyl glucosamine [10].
The polyol pathway (Aldose Reductase Enzyme)
Physiological Significance of aldose reductase: At present, physiological functions of aldose reductase have not been entirely clarified; its general role in most body tissues is to reduce toxic aldehydes in the cell to inactive alcohols, furthermore, some other specific roles have also been clarified [11].
Seminal energy production: The polyol pathway was first identified in the seminal vesicle by Hers, who demonstrated the conversion of blood glucose into fructose in seminal vesicle as an energy source for sperm cells [11].
Osmo-regulatory function in the kidney: Increased aldose reductase expression and accumulation of intracellular sorbitol in the cultured cell line from rabbit renal papilla as result of elevated extracellular sodium chloride was demonstrated[12] . In the renal medulla, mRNA of the enzyme was profusely expressed compared with relatively low cortex expression [13]. Therefore, these findings denote the osmo-regulatory role of aldose reductase in the renal homeostasis. Nevertheless, in non-renal cells, aldose reductase physiological osmo regulatory implications is still unknown.
Pathological roles of aldose reductase


Under normo-glycemic conditions, through hexokinase pathway, most of the cellular glucose undergoes phosphorylation into glucose 6-phosphate and smaller part of glucose (non- phosphorylated) enters the alternate route of glucose metabolism, the polyol pathway (Figure 2). The rate-limiting step of the polyol pathway is the reduction of glucose to sorbitol by aldose reductase enzyme, sorbitol dehydrogenase enzyme subsequently converts the produced sorbitol to fructose, thus constituting the polyol (sorbitol) pathway (Figure 3). When hexokinase is saturated by ambient glucose (under hyperglycemia), the flux of glucose through the polyol pathway then increased to account for as much as one-third of the total glucose turnover [14].
This leads to accumulation of the products of the polyol pathway that accompanied with depletion in reduced nicotinamide adenine dinucleotide phosphate (NADPH) as well as the oxidized form of nicotinamide adenine dinucleotide (NAD), the cofactors used in the pathway [15], it is significant to mention that NADPH is essential for regeneration of reduced glutathione (critical intracellular antioxidant) and nitric oxide (NO) synthase (NO is important for micro-vascular arrangement and nerve conduction)so, the polyol pathwayinduces intracellular hyper-osmolar pressure, increases susceptibility to intracellular oxidative stress , elicits micro-vascular derangement and slows the nerve conduction [16,17].
In rat as model, aldose reductase mRNA was highly expressed in the prime target organs of diabetic complications, the lens, the retina, and the sciatic nerve [13]. In the lens, the sorbitol accumulation induces cataract formation due to leakage of amino acids, glutathione, and myo-inositol because of hyperosmotic swelling and derangement of the cell membrane. In the retina of experimental models, the early lesion emerged in vascular component with localization of aldose reductase in retinal micro vessels [18-20]. In the nerves, perturbation in the vasculature and metabolic disturbance in the neural cells contributes to the development of diabetic neuropathy [21-23]. In fact, aldose reductase immuno reactivity was found in the paranodal cytoplasm of Schwann cells as well as in pericytes and endothelial cells of endoneurial capillaries [24]. Although insulin treatment effectively delay the onset of long term diabetic complications and slows their progression in patients with insulin-dependent diabetes mellitus (IDDM), it is practically impossible, even with best clinical management available, to maintain normo-glycemic state at all times throughout the life of diabetic individuals [25]. Accordingly, chemical agents that effectively halt the hyperglycemic injury in diabetic patients would be of great clinical importance.
Inhibitors of Aldose Reductase Enzyme
Depending on the previous observations, development of many aldose reductase inhibitors as possible therapeutic agents (with diverse chemical structures) for diabetic complications (to prevent retinal damage, cataracts, and nerve damage) became critical. The clinical efficacy of Sorbinil, ponalrestat, and tolrestat (the most studied inhibitors) in diabetic patients has not been fully proved to meet the standards of the Food and Drug Administration [26].
Synthetic aldose reductase inhibitors
Although many synthetic Aldose Reductase Inhibitors such as sorbinil and tolrestat exhibit potent inhibition, their use is now limited (or they have been withdrawn from clinical trials) because of decreased penetration, low efficacy, and safety problems) [27-29].
Sorbinil: When diabetic patients without any symptomatic neuropathy were treated with it, significant improvement in the velocity of conduction was observed in all nerves tested (the peroneal motor nerve, the median motor nerve, and the median sensory nerve) [30], because of the difference in the study design, subjects with various degrees of symptomatic neuropathy, and neuro physiological parameters examined as study endpoints, the overall effect turned out to be disappointingly modest [21].
Ponalrestat: Another aldose reductase inhibitor of a different chemical structure, with no clinically important adverse reaction observed (c.f. sorbinil), its beneficial effect failed to be proved in randomized controlled study [31], later, it was shown that itdidn't penetrate the human nerve at doses sufficient to decrease the nerve sorbitol levels [32].
Tolrestat: The efficacy of this class of inhibitor was the modest in diabetic patients already symptomatic of neuropathy, the only adverse reaction reported on it was an increase in serum levels of liver enzymes (alanine aminotransferase ALT, aspartate aminotransferase AST), clinical development of tolrestat was withdrawn, due to the inability to demonstrate efficacy on the nerve conduction velocity in the multicenter double-blindstudies on diabetic neuropathy [33].
Ranirestat: Is in Phase III trials in Europe and the US. Its clinical trial began in June 2009. The only available synthetic inhibitor is Epalrestat that is in Japanese market since 1992. So, there is still an urgent need for development of improved Aldose reductase inhibitors [34].
Natural Products as Potential inhibitors
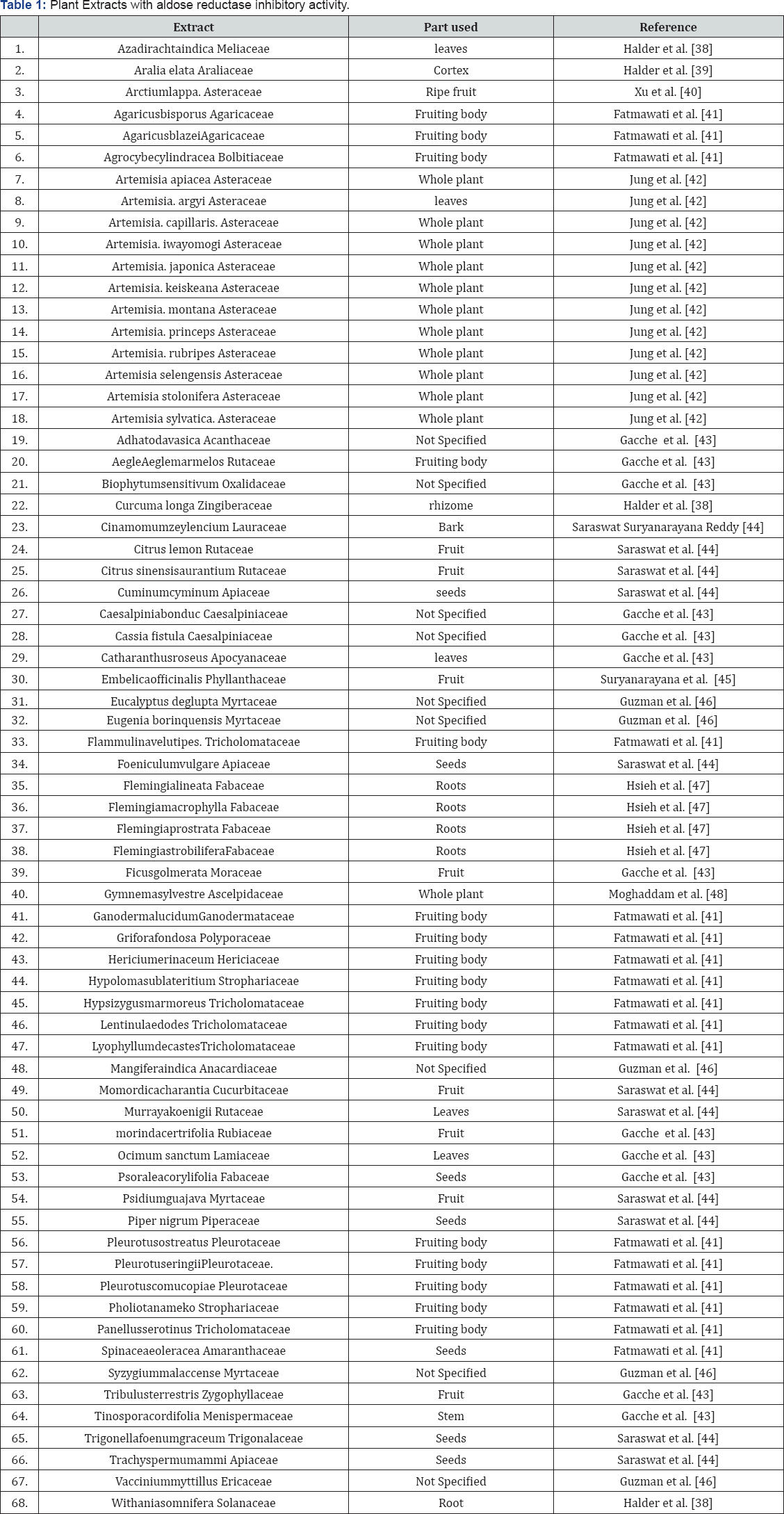

The benefits of dietary supplements such as naturceuticals and herbal medicines as pharmaceuticals have gain growing interest due to lack of toxicity and harmful side effects (they are daily consumed). Many structurally diverse phyto chemicals and extracts have been reported as potent aldose reductase inhibitors. Naturally occurring compounds with diverse chemical structures (flavonoids, coumarins tannins, alkaloids, terpenoids and phenolics) have significant aldose reductase inhibitory activity. Most of the natural sources either terrestrial or marinethat contain these compounds are presented in Table 1 & 2.
From natural sources that have been reported, Flavonoids and related compounds is the most widely studied natural product family with inhibitory activity. Vitamin C is one of the natural products that entered clinical trials, which showed 81% of in- vitro inhibition [35]. Vitamin C as dietary supplement seems to be effective in decreasing accumulation of erythrocyte's sorbitol and improves endothelium-dependent vasodilatation in diabetic patients [34-56].
Conclusion
Worldwide, researcher and scientists are widely interested in prevention of diabetic complications. Use of naturally occurring compounds in the treatment of variety of chronic disorders and illnesses is growing, and many extracts and isolated compounds are becoming better alternatives to synthetic drugs, diabetes and its complications can be prevented and/or decreased using these natural molecules. Quercet in Kaempferol and Ellagic acid is promising naturally occurring compounds that have evidences since a period for their aldose reductase inhibitory activity.
References
- Cooke DW, Plotnick L (2008) Type 1 diabetes mellitus in pediatrics. Pediatrics in Review / American Academy of Pediatrics 29(11): 374384.
- Kitabchi AE, Umpierrez GE, Miles JM, Fisher JN (2009) Hyperglycemic Crises in Adult Patients with Diabetes. Diabetes Care 32(7): 13351343.
- World Health Organization (2011) Diabetes Fact sheet No 312. WHO.
- Group TDC, CTR (1993) The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 329: 977-986.
- Heilig CW, Concepcion LA, Riser BL, Fraytag SO, Zhu M, et al. (1995) Over expression of glucose transporters in rat mesangial cells cultured in a normal glucose milieu mimics the diabetic phenotype. J Clin Invest 96(4): 1802-1814.
- Kaiser N, Sasson S, Feener EP, Boukobza VN, Higashi S, et al. (1993) Differential regulation of glucose transport and transporters by glucose in vascular endothelial and smooth muscle cells. Diabetes 42(1): 80-89.
- Gabbay KH, Merola LO, Field RA (1966) Sorbitol pathway: presence in nerve and cord with substrate accumulation in diabetes. Science 151(3707): 209-210.
- Baynes JW, Thorpe SR (2000) Glycoxidation and lipoxidation in atherogenesis. Free Radic Biol Med 28(12): 1708-1716.
- Koya D, King GL (1998) Protein kinase C activation and the development of diabetic complications. Diabetes 47(6): 859-866.
- Nishimura YC (1998) Aldose reductase in glucose toxicity: a potential target for the prevention of diabetic complications. Pharmacol Rev 50(1): 21-33.
- Hers HG (1956) Le mécanisme de la transformation de glucose en fructose par les vésicules séminales. Biochimica et Biophysica Acta 22(1): 202-203.
- Bagnasco SM, Uchida S, Balaban RS, Kador PF, Burg MB (1987) Induction of aldose reductase and sorbitol in renal inner medullary cells by elevated extracellular NaCl. Proc Natl Acad Sci USA 84(6): 1718-1720.
- Nishimura C, Graham C, Hohman TC, Nagata M, Robison WG, et al. (1988) Characterization of mRNA and genes for aldose reductase in rat. Biochem Biophys Res Commun 153(3): 1051-1059.
- Gonzalez RG, Barnett P, Aguayo J (1984) Direct measurement of polyol pathway activity in the ocular lens. Diabetes 33(2): 196-199.
- Lee AY, Chung SS (1999) Contributions of polyol pathway to oxidative stress in diabetic cataract. FASEB J 13(1): 23-30.
- Cameron NE, Cotter MA, Dines KC, Maxfield EK (1993) Pharmacological manipulation of vascular endothelium function in non-diabetic and streptozotocin-diabetic rats: effects on nerve conduction, hypoxic resistance and endoneurial capillarization. Diabetologia 36(6): 516522.
- Stevens MJ, Dananberg J, Feldman EL, Lattimer SA, Kamijo M, et al. (1994) The linked roles of nitric oxide, aldose reductase and, (Na+,K+)- ATPase in the slowing of nerve conduction in the streptozotocin diabetic rat. J Clin Invest 94(2): 853-859.
- Akagi Y, Kador PF, Kuwabara T, Kinoshita JH (1983) Aldose reductase localization in human retinal mural cells. Invest Ophthalmol Vis Sci 24(11): 1516-1519.
- Hohman TC, Nishimura C, Robison WG (1989) Aldose reductase and polyol in cultured pericytes of human retinal capillaries. Exp Eye Res 48(1): 55-60.
- Kennedy A, Frank RN, Varma SD (1983) Aldose reductase activity in retinal and cerebral microvessels and cultured vascular cells. Invest Ophthalmol Vis Sci 24(9): 1250-1258.
- Fagius J, Brattberg A, Jameson S, Berne C (1985) Limited benefit of treatment of diabetic polyneuropathy with an aldose reductase inhibitor: a 24-week controlled trial. Diabetologia 28(6): 323-329.
- Nishimura C, Lou MF, Kinoshita JH (1987) Depletion of myo-inositol and amino acids in galactosemic neuropathy. Journal of Neurochemistry 49(1): 290-295.
- Tomlinson DR, Moriarty RJ, Mayer JH (1984) Prevention and reversal of defective axonal transport and motor nerve conduction velocity in rats with experimental diabetes by treatment with the aldose reductase inhibitor sorbinil. Diabetes 33(5): 470-476.
- Chakrabarti S, Sima AAF, Nakajima T, Yagihashi S, Greene DA (1987) Aldose reductase in the BB rat: isolation, immunological identification and localization in the retina and peripheral nerve. Diabetologia 30(4): 244-251.
- Control TD, Trial C (1993) The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group The New England Journal of Medicine 329: 977-986.
- Pfeifer MA, Schumer MP, Gelber DA (1997) Aldose reductase inhibitors: The end of an era or the need for different trial designs? In Diabetes 46(2): S82-89.
- Kawanishi K, Ueda H, Moriyasu M (2003) Aldose reductase inhibitors from the nature. Curr Med Chem 10(15): 1353-1374.
- Manzanaro S, Salva J, De la Fuente JA (2006) Phenolic marine natural products as aldose reductase inhibitors. J Nat Prod 69(10): 1485-1487.
- Peyroux J, Sternberg M (2006) Advanced glycation endproducts (AGEs): pharmacological inhibition in diabetes. Pathol Biol (Paris) 54(7): 405-419.
- Judzewitsch RG, Jaspan JB, Polonsky KS, Weinberg CR, Halter JB, et al. (1983) Aldose Reductase Inhibition Improves Nerve Conduction Velocity in Diabetic Patients. N Engl J Med 308(3): 119-125.
- Krentz AJ, Honigsberger L, Ellis SH, Hardman M, Nattrass M (1992) A 12-Month Randomized Controlled Study of the Aldose Reductase Inhibitor Ponalrestat in Patients with Chronic Symptomatic Diabetic Neuropathy. Diabet Med 9(5): 463-468.
- Greene DA (1992) Effects of aldose reductase inhibitors on the progression of nerve fiber damage in diabetic neuropathy. In Journal of Diabetes and its Complications 6: 35-38.
- Boulton AJ, Levin S, Comstock J (1990) A multicentre trial of the aldose-reductase inhibitor, tolrestat, in patients with symptomatic diabetic neuropathy. Diabetologia 33: 431-437.
- De la Fuente JA, Manzanaro S (2003) Aldose reductase inhibitors from natural sources. Nat Prod Rep 20(2): 243-251.
- Cunningham JJ, Mearkle PL, Brown RG (1994) Vitamin C: an aldose reductase inhibitor that normalizes erythrocyte sorbitol in insulindependent diabetes mellitus. J Am Coll Nutr 13(4): 344-350.
- Cunningham JJ (1998) The glucose/insulin system and vitamin C: implications in insulin-dependent diabetes mellitus. J Am Coll Nutr 17(2): 105-108.
- Vincent TE, Mendiratta S, May JM (1999) Inhibition of aldose reductase in human erythrocytes by vitamin C. Diabetes Res Clin Pract 43(1): 1-8.
- Halder N, Joshi S, Gupta SK (2003) Lens aldose reductase inhibiting potential of some indigenous plants. Journal of Ethnopharmacology 86: 113-116.
- Chung YS, Choi YH, Lee SJ, Choi SA, Lee JH, et al. (2005) Water extract of Aralia elata prevents cataractogenesis in-vitro and in-vivo. Journal of Ethnopharmacology 101(1-3): 49-54.
- Xu Z, Yang H, Zhou M, Feng Y, Jia W (2010) Inhibitory effect of total lignan from Fructus Arctii on aldose reductase. Phytotherapy Research 24(3): 472-473.
- Fatmawati S, Kurashiki K, Takeno S, Kim YU, Shimizu K, et al. (2009) The inhibitory effect on aldose reductase by an extract of Ganoderma lucidum. Phytother Res 23(1): 28-32.
- Jung HA, Islam MDN, Kwon YS, Jin SE, Son YK, et al. (2011) Extraction and identification of three major aldose reductase inhibitors from Artemisia montana. Food Chem Toxicol 49(2): 376-384.
- Gacche RN, Dhole NA (2011) Profile of aldose reductase inhibition, anti-cataract and free radical scavenging activity of selected medicinal plants: An attempt to standardize the botanicals for amelioration of diabetes complications. Food Chem Toxicol 49(8): 1806-1813.
- Saraswat M, Muthenna P, Suryanarayana P, Petrash JM, Reddy GB (2008) Dietary sources of aldose reductase inhibitors: prospects for alleviating diabetic complications. Asia Pacific Journal of Clinical Nutrition 17(4): 558-565.
- Suryanarayana P, Kumar PA, Saraswat M, Petrash JM, Reddy GB (2004) Inhibition of aldose reductase by tannoid principles of Emblica officinalis: implications for the prevention of sugar cataract. Mol Vis 10: 148-154.
- Guzman A, Guerrero RO (2005) Inhibition of aldose reductase by herbs extracts and natural substances and their role in prevention of cataracts. Rev Cubana Plant Med 10: 1-7.
- Hsieh PC, Huang GJ, Ho YL, Lin YH, Huang SS, et al. (2010) Activities of antioxidants, a-Glucosidase inhibitors and aldose reductase inhibitors of the aqueous extracts of four Flemingia species in Taiwan. Botanical Studies 51: 293-302.
- Moghaddam MS, Kumar PA, Reddy GB, Ghole VS (2005) Effect of Diabecon on sugar-induced lens opacity in organ culture: Mechanism of action. J Ethnopharmacol 97(2): 397-403.
- Endo M, Nakagawa M, Hamamoto, Y, Ishihama M (1986) Pharmacologically active substances from southern pacific marine invertebrates. Pure and Applied Chemistry 58(3): 387-394.
- Nakagawa M, Ishihama M (1987) Tennen Yuki Kagobutsu Toronkai Koen Yoshishu 29: 552-559.
- Sugano M, Sato A, Nagaki H, Yoshioka S, Shiraki T, et al. (1990) Tetrahedron Lett 31: 7015.
- Nakamura H, Yamaguchi S, Hayashi T, Baba M, Okada Y, et al. (1997) Nat Med 51: 162.
- Gacche RN, Dhole NA (2011) Aldose reductase inhibitory, anti-cataract and antioxidant potential of selected medicinal plants from the Marathwada region, India. Nat Prod Res 25(2): 760-763.
- Nagata M, Hohman TC, Nishimura C, Drea CM, Oliver C, et al. (1989) Polyol and vacuole formation in cultured canine lens epithelial cells. Exp Eye Res 48(5): 667-677.
- Sato A, Morishita T, Shiraki T, Yoshioka S, Horikoshi H, et al. (1993) Aldose reductase inhibitors from a marine sponge, Dictyodendrilla sp. The Journal of Organic Chemistry 58(27): 7632-7634.
- Turner R (1998) Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 352(9131): 854-865.






























