Role of Amniotic Membrane Transplantation in Symblepharon
Ahmed Tamer Sayed Saif1*, Mohamed Osman Abdel Khalek2 and Waleed Mohamed Mahran2
1Ophthalmology Department, Fayoum University, Egypt
2Ophthalmology Department, Beni-Suef University, Egypt
Submission: April 27, 2016; Published: June 8, 2016
*Corresponding author: Ahmed Tamer Sayed Saif, Ophthalmology department, Fayoum University, 5 sherif st, Babel Louk sq, Fayoum, Cairo 11111, Egypt, Email:ysaif@med.bsu.edu.eg.
How to cite this article: Ahmed Tamer S S, Mohamed Osman A K, Waleed M M. Role of Amniotic Membrane Transplantation in Symblepharon. JOJ Ophthal. 2016; 1(3): 555565. DOI:10.19080/JOJO.2016.01.555565
Abstract
Aim: To evaluate the use of amniotic membrane transplantation (AMT) in symblepharon.
Methods: This non comparative interventional case study was conducted from January 2013 to December 2015 and included a consecutive series of 14 eyes of 12 patients. Patients were selected for permanent AMT. The amniotic patches were grafted for the treatment of symblepharon. Cryo-preserved or freeze-dried amniotic membrane (AM) was used. Regarded to the 14 eyes (12 patients), their age were ranged from 26-62y, with the mean age of 43.38 ± 11.25, 10 eyes of 8 patients (4M/ 4F) were presented with symblepharon secondary to previous pterygium surgery, and 4 eyes of 4 patients (1M/ 3F) were presented with symblepharon secondary to previous strabismus surgery, at least 6 months after the last surgery. The outcome of success was defined as restoration of a stable-depth fornix and being free of scar or inflammation, and no motility restriction during the follow up of 6 months.
Results: The mean follow-up period was 7 ± 4.2 months (range, 6–9 months). In all 14 eyes, complete epithelialization of AM was observed 3 weeks after surgery, resulting in a non-inflamed appearance of the surgical site. Eight eyes out of total 14 eyes showed successful fornix reconstruction with success rate (57%), the fornix was deep, and no recurrence was observed. Four eyes (28.75%) showed partial success with moderate depth of the fornix and moderate scar. Two eyes (14%) showed failure of reconstruction of the fornix with complete fornix obliteration. The visual acuity improved after surgery in seven eyes while remained stable in seven eyes. Post operative complications from the AMT was very limited as severe conjunctively reaction and motility restriction was occurred only in one eye out of 14 eyes(7.1%) and phylogenic granuloma occurred in 2 eyes out of 14 eyes (14.2%) in the first 3 months after surgery and was managed with surgical excision, with local corticosteroid injection .
Conclusion: AMT alone is a safe and effective method for symblepharon. Considering the potential adverse effects associated with limber excision, also, AMT is an effective method of fornix reconstruction for the repair of symblepharon in a variety of ocular surface disorders.
Keywords: Amniotic membrane transplantation; Symblepharon; Pterygium; Strabismus; Fornix reconstruction
Abbreviations: AMT: Amniotic Membrane Transplantation; AM: Amniotic Membrane; MUC: Mucin; LSCD: Limber Stem Cell Deficiency; AM: Amniotic Membrane; ELISA: Enzyme Linked Immune Sorbant Assay; PCR: Polymerase Chain Reaction; HIV: Human Immunodeficiency Virus; SPSS: Statistical package for social science; LSCD: Limber Stem Cell Deficiency
Introduction
Conjunctively epithelium is non-keratinized consists of two phenotypically distinct cell types, stratified squamous non-goblet cells (90%-95%) and goblet cells (5%-10%) , in addition to occasional lymphocytes and melanocytes. The conjunctively epithelium plays an important role in ensuring the optical clarity of the cornea by providing lubrication to maintain a smooth, refractive surface, and by producing mucus critical for tear film stability [1]. The conjunctiva also protects the eye against mechanical stress and infectious agents. It furthermore, contributes water and electrolytes to the tear fluid. The squamous cells produce cell membrane-tethered mucus, while the goblet cells secrete the gel-forming mucus, both of which help to maintain a protective tear film. The superficial surface of the squamous cells are covered by the membrane-tethered mucus mucin-1 (MUC1), mucin-4 (MUC4) and mucin-16 (MUC16) , which are essential for tear stability and make up the glycocalyx [2].
Conjunctively stem cells continuously regenerate the conjunctiva by giving rise to both stratified squamous non-goblet and goblet cells, thereby maintaining a healthy tear film. Disorders that damage these stem cells cause varying extent of keratinization, which disrupts the protective tear film and ultimately leads to limber stem cell deficiency (LSCD) and visual impairment or blindness. The location of the conjunctively epithelial stem cells has been investigated in several studies on mouse, rat, rabbit, and human tissue, yet no real consensus has been reached. The conjunctively stem cells have been suggested to reside in the limbus bulbar conjunctiva, medial Canthus, forniceal conjunctiva, palpebral conjunctiva and mucocutaneous junction [3].
Although the conjunctively stem cells may not solely be located to one single region, their relative number generally appears to be highest in the fornix. A large number of disorders can lead to scarring of the conjunctiva through chronic conjunctively inflammation. Scarring varies in severity and can be self-limited, such as in chemical/thermal burns , iatrogenic for example postoperative as in recurrent pterygium surgeries or post strabismus surgeries and infectious diseases due to adeno- and herpes viruses, or progressive, as in cicatrizing conjunctivitis, which consists of several diseases including ocular cicatricial pemphigoid, Stevens-Johnson syndrome, atopic keratoconjunctivitis and Sjögren’s syndrome [4]. Treatment depends on the disease etiology and severity, but can include various anti-inflammatory, immunomodulatory and immunosuppressive drugs. One of the most common complications of scarring of the conjunctiva is symblepharon formation where there is adhesion between the bulbar and palpebral conjunctiva. Surgical treatment of symblepharon includes removal of the scar tissue and the surgical defect is then covered with a tissue substitute to prevent re-obliteration. These include mechanical physical or chemical approaches and the grafting of conjunctively or mucous membranes [5]. Surgical techniques for restoration of a diseased conjunctiva have utilized different Conjunctively substitutes, including Conjunctively auto grafts , amniotic membrane (AM), buccal mucosal grafts and tissue engineered Conjunctively epithelial equivalents . An obvious limiting factor when using auto grafts is the size of the defect to be covered, as the amount of healthy conjunctiva is limited [6,7]. AM was first used in ophthalmology seventy-six years ago. AM constitutes the innermost layer of the fetal membranes. AM is particularly suited for clinical use as it supports epithelialization , reduces scaring , suppresses the immune response , reduces pain, and decreases inflammation [8-10].
AM’s are commercially available in two forms either cryopreserved or freeze-dried. In cryopreserved type, the AM cryopreserved in a basal cell medium at -80 °C. Hence it kills all the AM cells, so the AM grafts function primarily as a matrix and not by virtue of transplanted functional. The Freeze-dried AM can be sterilized by gamma-irradiation; however, AM treated this way may release a less amount of growth factors than conventionally cryopreserved membranes [11,8].
Subject and Materials
This non comparative interventional case study was conducted from January 2011 to December 2015 and included a consecutive series of 14 eyes of 12 patients. The study was approved by the institutional review board and was conducted in accordance with the principles of the Declaration of Helsinki. Patients were selected for permanent AMT. The amniotic patches were grafted for the treatment of symblepharon. Cryo-preserved or freeze-dried amniotic membrane (AM) was used. Regarded to the 14 eyes (12 patients), their age were ranged from 26-62y, with the mean age of 43.38y (SD: 11.25), 10 eyes of 8 patients (4M/ 4F) were presented with symblepharon secondary to previous pterygium surgery, and 4 eyes of 4 patients (1M/ 3F) were presented with symblepharon secondary to previous strabismus surgery, at least 6 months after the last surgery.
Inclusion criteria
All patients were presented with post surgical symblepharon after at least 6 months from last operation.
Exclusion criteria
1) Post surgical symblepharon before 6 months after the last operation; 2) Patients with previous corneal perforation (as indicated by entangled iris tissue in the wound of previous operation); 3) Symblepharon secondary to immune diseases; 4) Patients with Punctual occlusion , and +ve regurgitation from the punctum.
All the patients were subjected to the following
Full history includes: name, age, gender, duration of the disease, past history of previous ocular surgeries, or trauma to the affected eye and medical history of previous medications either topical or systemic. The patients were submitted to full medical examination for any possible systemic disorders. (E.g. diabetes, hypertension and neurological disorders). Ophthalmological examination of the patients was carried out using slit-lamp biomicroscopy (SHIN NIPPON—JAPAN) including; morphological appearance of the lesion, affection of medial rectus muscle and any associated symblepharon or corneal scars. Examination of the corneal epithelium, stroma and the endothelium. Epithelial defect was examined as regard site, size, shape, number, depth and edge. Diagnostic dyes, as fluoresce in or Rose Bengal, were used, as well as determination of the break up time and Schirmer’s tests were also carried out. Corneal sensitivity was assisted by using twisted tip of a cotton piece touching the central corneal zone of the patient. Serological tests including conjunctively smear and corneal scraping for culture and sensitivity test.
Visual acuity was tested using Snellen chart and intraocular pressure measurement by applanation tonometer (SHIN NIPPON-JAPAN). Fundus examination using indirect ophthalmoscope (KEELER-ENGLAD). Regurgitation test were carried in every patient by pressing at the site of the lachrymal sac. Upper and lower lids, lachrymal glands and puncti were assessed.
Amniotic membrane preparation
Protocol of tissue preparation:Selection of the donor: A detailed medical and behavioral history together with signed consent was obtained from the donor mother. Serological tests were carried out by both (ELISA) and (PCR) for HIV, hepatitis B, C. Under a lamellar flow hood, the placenta obtained shortly after caesarean delivery, was first washed, free of blood clots with balanced saline solution containing50 μg/ml of penicillin, 50 μg/ml of streptomycin, 100 μg/ml of neomycin, and 2.5 μg/ ml of amphotericin B (Fungi zone 50 mg Bristol Myers Squibb). The inner amniotic membrane was separated from the rest of the chorionic by blunt dissection (through the potential spaces between these two tissues). The membrane was then cut into 4 × 4 cm pieces and placed in a sterile vial containing Dulbecco’s Modified Eagle’s medium and glycerol ata ratio of 1:1 (vol/ vol). For cryopreserved AM, the vials were frozen at 80°C to be used within less than a month. The membrane was defrosted immediately before use by warming, the container to room temperature for 10 minutes.
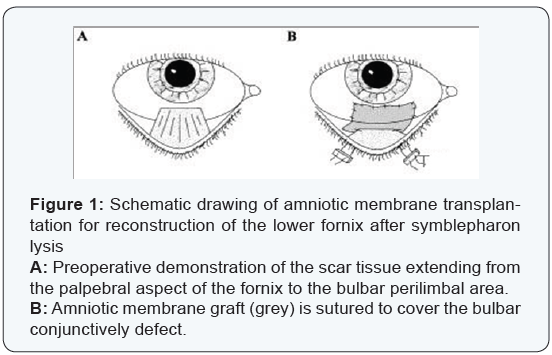
Surgical techniques:All patients were anesthetized with a peri-bulbar block. . Eyes with severe symblepharon obliterating the fornix to the extent that the speculum could not be inserted, one 4-0 black silk suture was placed at the lid margin through the tarsal plate of each lid as a traction. Those eyes in which symblepharon were not severe were opened with a lid speculum. Once the eye was opened, the conjunctiva was incised from the peri-limber region between the normal conjunctiva and the beginning of the cicatrix. If the cicatrix was focal, such an incision was made for <180° and relaxing incisions were then made at the border of the cicatrix toward the fornix. For traction suture 7-0 Vicryl sutures were used at the superior or inferior limber sclera to redirect the globe, so that the cicatrix could be better exposed. Meticulous dissection by scissors was then done to separate and remove the entire sub conjunctively fibro vascular tissue in each bulbar conjunctiva up to the fornix in each quadrant. After the globe was rotated by the 7/0 traction suture and the removal of cicatrix, the incised conjunctively edge was invariably and naturally recessed to the deep fornix or the tarsus. The AM was thawed and laid down on the bare sclera with the stromal side facing down. If the symblepharon was focal and fornix shortening was limited, the membrane was secured to recessed conjunctiva by an interrupted 8-0 Vicryl suture with episcleral bites, (Figure 1). If the symblepharon was diffuse and fornix shortening involved nearly the entire fornix, the membrane was secured to the recessed conjunctiva by a running 8-0 Vicryl suture and the graft was pushed to the deep fornix by a muscle hook and anchored there by passing one double-armed 4-0 black silk suture per quadrant through the full thickness of the lid and securing it to the skin with a bolster made of silicone tubing, (Figure 2).The remaining AM was then flattened and secured on the bare sclera by interrupted 8-0 Vicryl sutures with long episcleral bites. Care was taken to avoid trapping blood under the membrane.
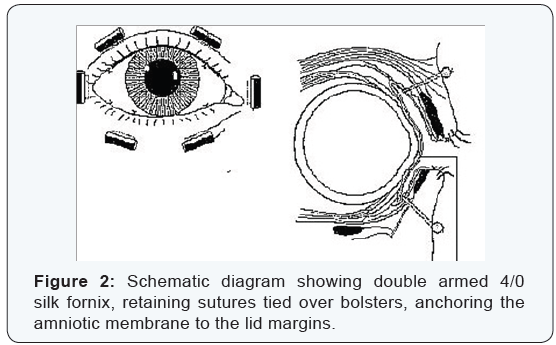
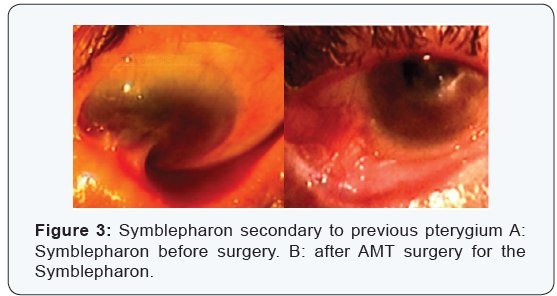
Postoperative evaluation and follow up:Postoperatively all patients received topical combination of tobramycine and dexamethasone eye drops 5 times daily and Tobradex eye ointment twice daily, and tapered off in a period of 4 to 6 weeks, until full epithelialization of the AM was evident, as determined on the first postoperative visit when no fluorescent staining was demonstrated over the amniotic membrane. Sutures were usually removed at the 2nd week postoperatively. The outcome of success was defined as restoration of a stable-depth fornix and being free of scar or inflammation, and no motility restriction during the follow up of 6 months (Figure 3). Partial success was defined as focal recurrence of scar tissue without inflammation, whereas failure was defined as the return of inflamed and scarred tissue in the area of surgery and obliteration of the fornix at the last follow up period.
Statistical analysis:Collected data were entered into an excel sheet and stored in excel format using Microsoft Excel 2007.For statistical analysis, Statistical package for social science (SPSS) software version 17 was used. The following tests were used: descriptive analysis of the results in the form of percentage distribution for qualitative data (minimum, maximum, mean and standard deviation) calculation for quantitative data. Cross tabulation test: for comparison between percentage values. Student t- test: For comparison between means of two groups. F- Test (One way ANOVA): a test statistics calculated for comparison between means of three groups. The Correlation coefficient (r): A correlation is a single number that describes the degree of relationship between two variables. The most common type, called the Pearson correlation. P values less than 0.01 were considered significant. The sign of the correlation coefficient (+, -) defines the direction of the relationship, either positive or negative. A positive correlation coefficient means that as the value of one variable increases, the value of the other variable increases; as one decreases the other decreases. A negative correlation coefficient indicates that as one variable increases, the other decreases, and vice-versa.
Results
Fourteen eyes of 12 patients (5M /7F) were presented with symblepharon. The mean follow-up period was 7 ± 4.2 months (range, 6-9 months). In all 14 eyes, complete epithelialization of AM was observed 3 weeks after surgery, resulting in a noninflamed appearance of the surgical site. Complete success was defined as achievement of deep fornix with no obliteration and no limitation of ocular motility .While failure is determined as complete obliteration of the fornix with extreme limitation of the ocular motility at the same direction of symblepharon. Partial success is defined as any degree of adhesion or limitation of ocular motility in-between the complete remission and complete failure. Eight eyes out of total 14 eyes showed successful fornix reconstruction with success rate (57%), the fornix was deep, and no recurrence was observed. Four eyes (28.75%) showed partial success with moderate depth of the fornix and moderate scar. Two eyes (14%) showed failure of reconstruction of the fornix with complete fornix obliteration .The visual acuity improved after surgery in seven eyes while remained stable in seven eyes (Table 1). Post operative complications from the AMT was very limited as severe Conjunctively reaction and motility restriction was occurred only in one eye out of 14 eyes(7.1%) and phylogenic granuloma occurred in 2 eyes out of 14 eyes ( 14.2%) in the first 3 months after surgery and was managed with surgical excision, with local corticosteroid injection Ten eyes of symblepharon secondary to previous pterygium surgery (4M, 4F, mean age 43.38 years) showed successful fornix reconstruction in 6 eyes (3M, 2F), the fornix was deep, and no recurrence was observed. Two eyes (1M, 1F) showed partial success with moderate depth of the fornix and moderate scar. Two eyes (1M, 1F) showed failure of reconstruction of the fornix with complete fornix obliteration. Four eyes of symblepharon secondary to previous strabismus surgery, (1M, 3F, mean age 31.5 years) showed successful fornix reconstruction in 2 eyes (1M, 1F), with no recurrence in the follow up period (6 months). Partial success with moderate fornix depth was observed in 2 eyes (2F) in the last follow up time. There is no statistical difference in the recurrence rate and the complications between the cryopreserved AM and freeze-dried AM
Discussion
Amniotic membrane (AM), the innermost layer of fetal (or placental) membrane, consists of a thick basement membrane and an avascular stroma. Its function is to protect the fetus from unwanted maternal insults during development. It has been commonly recognized that the incision made via the skin of the fetus during fetal surgery performed in the third trimester does not bear any scarring after birth. The phenomenon of “scar-less fetal wound healing” remains to be elucidated. It is not clear if AM carries the same feature as the fetal tissue [12]. There are a number of action mechanisms that may explain the effectiveness of AM used in ocular surface reconstruction: The AM’s basement membrane facilitates migration of epithelial cells, reinforces adhesion of basal epithelial cells, and promotes epithelial differentiation, and prevent epithelial apoptosis.The AM’s stroma contains growth factors, anti-angiogenic and antiinflammatory proteins [13]. The formation of symblepharon may destabilize the tear film by interfering with eyelid blinking and tear meniscus formation, by reducing the size of goblet cell– containing conjunctiva, by facilitating mechanical trauma caused by lid malposition and misdirected lashes, and by limiting the ocular motility. Symblepharon as such further aggravates the underlying pathology and directly accounts for the patient’s symptoms of discomfort. Furthermore, without being first corrected, such symblepharon is a major obstacle, if not a contraindication, for the ensuing corneal transplantation and ocular surface reconstruction [14,15].
When a large symblepharon is surgically removed, the Conjunctively defect is normally healed by the surrounding conjunctiva with granulation and scarring, which may lead to disfiguring and motility restriction of the extraocular muscles or the lid blinking. To avoid such potential problems, Conjunctively autograft from the same eye or the fellow eye is frequently used. However, some patients might not have healthy Conjunctively tissue to spare and further removal of the uninvolved conjunctiva might put the patient at additional risks .Procedures of symblepharon correction are numerous, suggesting that each variation has its limitations. Conventional therapies should possess the dual actions of restoring the normal Conjunctively tissue in the symblepharon-lysed area and preventing the underlying cicatricial processes leading to re-adhesion [16,17].
In this study, AMT was used in 14 eyes of 12 patients with symblepharon, secondary to previous surgery( 10 eyes had symblepharon secondry to previous pterygium surgery & 4 eyes secondry to previous strabismus surgery ) . The mean followup was 6.4 months. Cryoperserved or dired AM was applied alone after lysis of symblepharon and release of the adherent conjunctiva.The degree of success of the outcome was measured by formation of a deep fornix, with complete epithelialization of AM without inflammation and partial improvement of the ocular motility. This was achieved in 8 eyes of the 14 eyes (57%). Partial success was reported in 4 eyes (28.57%), in which moderate fornix depth was achieved with moderate ocular motility, but without inflammation or severs scarring. Failure was reported in 2 eyes (14%) in which persistence of adhesion between the lid and the bulbar conjunctiva still present with very shallow fornix and restricted ocular motility. In these cases the host scar tissue was not removed but rather recessed to the fornix after symblepharon lysis. Thus, the remaining scar tissue may be responsible for the failure in these patients. Ten eyes of symblepharon secondary to previous pterygium surgery (4M, 4F, mean age 43.38 years) were included in this study. Six eyes (60%) (3M, 2F), showed successful fornix reconstruction. The fornix was deep, and no recurrence was observed.
Two eyes (20%) (1M, 1F) showed partial success with moderate depth of the fornix and moderate scar. Two eyes (20%) (1M, 1F) showed failure of reconstruction of the fornix with complete fornix obliteration. Four eyes of symblepharon secondary to previous strabismus surgery were including in this study, (1M, 3F, mean age 31.5 years). Two eyes (50%) (1M, 1F) showed successful fornix reconstruction, with no recurrence in the follow up period (6 months). Partial success with moderate fornix depth was observed in 2 eyes (50%) (2F) in the last follow up time. Solomon et al. [18], did a retrograde study series on 80 eyes with postoperative restrictive strabismus (post pterygium, retinal detachchment, orbital floor fracuture, dermoid cyst and strabismus surgieries) to evaluate the sucess rate of AMT in treatment ocular misaligment and restore orthophoria and normal ocular motility. This goal acheived in 87% of the cases. These results are higher than ours, as the sucesss rate is defined as acheivement of complete fornix reconstction compared to this study where sucess rate is achevied only after orthophoria and free ocular motility. In 2001, Abraham Nakamura T et al. [19] did a study to evaluate AMT for fornix reconstruction after symblepharon due to pervious ocular surface disorders. Seventeen eyes were included in this study. Four eyes had ocular-cicatricial pemphigoid, two eyes had symblepharon after pterygium excision, four eyes had chemical or mechanical trauma, two eyes had strabismus surgery, two eyes (one patient) had Stevens-Johnson syndrome, one eye had toxic epidermal necrolysis, and two eyes (one patient) had chronic allergic conjunctivitis. The mean follow-up period was ranged 9-24 months. Complete fornix reconstruction was demonstrated in 12 of 17 eyes (70.6%), whereas 2 eyes had a partial success, and 3 eyes (3 patients) had recurrence of symblepharon with restricted motility. In eyes that demonstrated partial success or failure, the underlying etiology was either an autoimmune disorder or chemical burn. The most successful outcome was observed in eyes with symblepharon associated with previous pterygium or strabismus surgeries (69.4 %).
These results are compatible with our results Tseng T et al. [20], 2001, reported successful fornix reconstruction in 9/10 eyes with symblepharon using sterilized, freeze-dried amniotic membrane (FD-AM)with a mean follow up period of 13.5 ± 3.8 months. The complete healing took 1 to 6 weeks. These results are matching to our results. Tseng T et al. [20], 1997, had no comparative interventional study to investigate the role of AMT with application of mitomycin C for fornix reconstruction in cicatricial ocular surface diseases. Sixteen patients (8 female, 8 male; 18 eyes) with a mean age of 41±23.4 years (range, 3-79) and suffering from severe chemical/thermal burns (7 eyes), multiple recurrent pterygium and pseudopterygia (5 eyes), Stevens- Johnson syndrome (4 eyes), and ocular cicatricial pemphigoid (2 eyes) were consecutively enrolled. All except for 2 eyes had prior surgical attempts of surgical reconstruction, including 6 eyes with a mucous membrane graft (MMG), but still presented with symblepharon and persistent ocular surface inflammation. The mean epithelial healing time was 4.2±1.9 weeks. The best results achieved in with eyes with post pterygium symblepharon with success rate (65%) and the least with previous chemical burns (28%). The success rate in this study is matching with our study results but the mean epithelial healing time in this study (4.2 ±1.9M)is delayed compared to our study ( 3 w) ,this is due to the application of MMC. In conclusion AMT alone is a safe and effective method for symblepharon. Considering the potential adverse effects associated with limber excision, also, AMT is an effective method of fornix reconstruction for the repair of symblepharon in a variety of ocular surface disorders. Future modifications, including an epithelial cellular component on the AM (Conjunctively autography or ex vivo expanded epithelial stem cells) may improve the outcome of this surgical procedure. AM can be used in other pathologies rather than included in this study. It can be used in glaucoma under the flap in trabeculectomy operation. Also, for reconstruction of shallow fornices in contracted socket. Again, it helps in increasing success of dacryocystorhinostomy operation.
References
- Ramos T, Scott D, Ahmad S (2015) An update on ocular surface epithelial stem cells: Cornea and conjunctiva. Stem Cells Int 2015: 601731.
- Gendler SJ, Spicer AP (1995) Epithelial mucin genes. Annu Rev Physiol 57: 607-634.
- Stewart RM, Sheridan CM, Hiscott PS, Czanner G, Kaye SB (2015) Human conjunctival stem cells are predominantly located in the medial canthal and inferior forniceal areas. Invest Ophthalmol Vis Sci 56(3): 2021-2030.
- Radford CF, Rauz S, Williams GP, Saw VP, Dart JK (2012) Incidence, presenting features, and diagnosis of cicatrising conjunctivitis in the United Kingdom. Eye 26(9): 1199-1208.
- Sobolewska B, Deuter C, Zierhut M (2013) Current medical treatment of ocular mucous membrane pemphigoid. Ocul Surf 11(4): 259-266.
- Donnenfeld ED, Perry HD, Wallerstein A, Caronia RM, Kanellopoulos AJ, et al. (1999) Subconjunctival mitomycin C for the treatment of ocular cicatricial pemphigoid. Ophthalmology 106(1): 72-79.
- El Gendy NMS (2013) Use of amniotic membrane grafting to cover surgically induced superficial keratectomy during pterygium excision surgery. J Egypt Ophthalmol Soc 106(1): 50-53.
- Shashikala P (2013) Is amniotic membrane transplantation, an adjuvant of choice following excision of primary Pterygium. J Clin Ophthalmol Res 1(2): 91-93.
- Fein W (1979) Repair of total and subtotal symblepharons. Ophthalmic Surg 10(6): 44-47.
- Okoye O, Oguego NC, Chuka Okosa CM, Ghanta M (2013) Short term results of pterygium surgery with adjunctive amniotic membrane graft. Niger J Clin Pract 16(3): 356-359
- Solomon A, Espana EM, Tseng SC (2003) Amniotic membrane transplantation for reconstruction of the conjunctival fornices. Ophthalmology 110(1): 93-100.
- Sinha R, Tinwala SI, Shekhar H, Titiyal JS (2013) Amniotic membrane transplantation in ocular surface disorders: A review. J Clin Ophthalmol Res 1(1): 64-69.
- Ueta M, Kweon MN, Sano Y, Sotozono C, Yamada J, et al. (2002) Immunosuppressive properties of human amniotic membrane for mixed lymphocyte reaction. Clin Exp Immunol 129(3): 464-470.
- Prabhasawat P, Tesavibul N, Prakairungthong N, Booranapong W (2007) Efficacy of amniotic membrane patching for acute chemical and thermal ocular burns. J Med Assoc Thai 90(2): 319-326.
- Tanaka TS, Demirci H (2016) Cryopreserved Ultra-Thick Human Amniotic Membrane for Conjunctival Surface Reconstruction after Excision of Conjunctival Tumors. Cornea 35(4): 445-450.
- Tseng SCG (2001) Amniotic membrane transplantation for ocular surface reconstruction. Biosci Rep 21(4): 481-489.
- Strube YN, Conte F, Faria C, Yiu S, Wright KW (2011) Amniotic membrane transplantation for restrictive strabismus. Ophthalmology 118(6): 1175-1179.
- Solomon A, Espana EM, Tseng SC (2003) Amniotic membrane transplantation for reconstruction of the conjunctival fornices. Ophthalmology 110(1): 93-100.
- Nakamura T, Yoshitani M, Rigby H, Fullwood NJ, Ito W, et al. (2004) Sterilized, freeze-dried amniotic membrane: a useful substrate for ocular surface reconstruction. Invest Ophthalmol Vis Sci 45(1): 93- 99.
- Tseng SC, Di Pascuale MA, Liu DT, Gao YY, Baradaran-Rafii A (2005) Intraoperative mitomycin C and amniotic membrane transplantation for fornix reconstruction in severe cicatricial ocular surface diseases. Ophthalmology 112(5): 896-903.







