The Flash Electroretinogram of Burrowing Owl – Athene cunicularia
Márcio Penha Morterá Rodrigues1, Luiz Reis Barbosa Júnior1*, Antônio Luiz Zangalli1, Eduardo de Franca Damasceno2 and Adalmir Morterá Dantas1
1Carlos Chagas Filho Biophysics Institute, Federal University of Rio de Janeiro (UFRJ), Brazil
2Fluminense Federal University (UFF), Brazil
Submission: January 13, 2016; Published: February 01, 2016
*Corresponding author: Luiz Reis Barbosa Junior, Carlos Chagas Filho Biophysics Institute, Faculty of Medicine of Health Sciences Center, Federal University of Rio de Janeiro (UFRJ), Av. Pedro Calmon 550, University City, Rio de Janeiro, 21941-901, RJ, Brazil; Email: luiz_reis@hucff.ufrj.br
How to cite this article: Rodrigues MPM, Reis L, Zangalli AL, Dantas AM. The Flash Electroretinogram of Burrowing Owl – Athene cunicularia. JOJ Ophthal. 2016; 1(3): 555561. DOI:10.19080/JOJO.2016.01.555561
Abstract
Background: The burrowing owl (Athene cunicularia) has peculiar visual capacity, presenting conspicuous vision, what can be justified, among other motives, by the presence of cones and rods in the macula and the existence of double cones. The Athene cunicularia presents retinal cell circuit highly organized and complex, with presence of two foveae. Thickening in the ganglion cell layer surrounding the fovea contributes to the change of the refractive index of the retina at this location, thus increasing the sensitivity for detecting motion. The high concentration of photoreceptors in the fovea is the cause of the maximum optical resolution of this region.
Results: We conduct a transversal observational study according to the protocol of the International Society for Clinical Electrophysiology of Vision (ISCEV) that aims at evaluating the retinal response of the species after stimulation with white light, both in photopic and scotopic stimuli. The verification of the p-value and t-value, we obtained for the variables, respectively: a-wave amplitude - 0.02 and 2.94; b-wave amplitude - 0.12 and 1.76; b-wave time culmination - 0.07 and 2.12;b-wave latency - 0.74 and 0.34.
Conclusion:In order to standardize the variables of the electroretinogram of the species, the authors propose to evaluate the electroretinogram components under white light in both stimuli, providing electrophysiological data from the visual stimulation of retinal species, encouraging the Biological Sciences with substrate for future research and clinical evaluations.
Keywords: Owls; Electroretinography; Retina
Abbreviations: CEPAL: Ethics Committee of Faculty of Medicine of Health Sciences Center in Federal University of Rio de Janeiro; ERG: Electro Retino Gram; IBAMA: Brazilian Institute of Environment and Renewable Natural Resources; ISCEV: International Society for Clinical Electrophysiology of Vision
Introduction
The retinal electrical activity corresponds to the initial event processing of visual information. All animal species that have visual apparatus present some retinal response, so that the comparative study of different species allows us to acquire knowledge that can be applied in our daily lives.
It is true that most treatments used in humans result from applications arising from animal research. Knowledge of cytology, histology and physiology of the human race is due to the various animal studies.
The owls are known for their great visual capacity due to their complex retinal structure. As an interesting kind of vertebrates, the investigation of their visual apparatus operation is of most importance. The existence of a previous study on the morphology of photoreceptors and the evaluation of the b-wave of electroretinogram (ERG) in various color spectra in Athene cunicularia (Burrowing Owl) motivated acomplementary electroretinographic study of the specie concerned [1].
The visual capacity of owls presents peculiarities, among them, their big eyes which account for 1-5% of body weight, depending on the species. They have binocular vision, which provides them stereopsis. Their total visual field is 110°, having 70° binocular vision. Their eyes are large to increase their efficiency, especially under low light conditions and are so well developed that they are not rounded, but tubular. They are protected by three structures, the upper eyelids, lower and the nictitating membrane [2].
The retina of Athene cunicularia has mixed fovea, with a predominance of rods, observing the eight rods relationship for two simple cones and a double cone [3-5]. The presence of a double cone only occurs in some species [6-8]. Another important morphological appearance in the retina of these birds (including the Athene cunicularia) is the presence of aster, a structure mainly composed of axons of bipolar cells that have oblique direction and centrifugal to the fovea, allowing for a retinal neuronal rearrangement circuit area resulting from the central area [6,9-11].
The Athene cunicularia presents a retinal cell circuit extremely organized and complex. Thickening in the ganglion cell layer surrounding the fovea contributes to the change of the refractive index of the retina at this location, thus increasing the sensitivity for movement’s detection [12-15]. The high concentration of photoreceptors in the fovea is what accounts for the maximum optical resolution of this region [3]. The Athene cunicularia does not present differentiated retinal electrical response to some light spectra in photopic and scotopic environment, when taking into account the latency and amplitude of b-wave [1].
In order to standardize the variables of the electroretinogram of the species, the authors, following their line of research, have proposed to evaluate the electroretinogram components under white light in both environments (Figure 1 and 2).
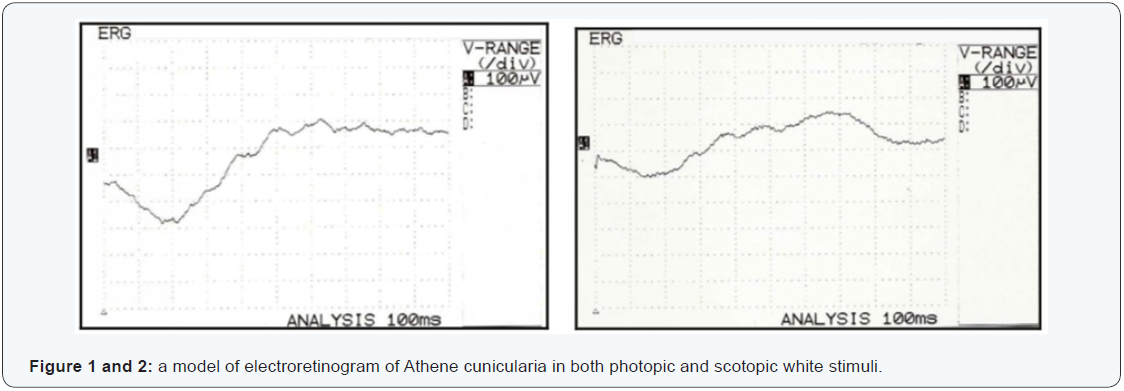
The Athene cunicularia has twilight hunting habits and previous histological descriptions identified outer segments of the long rods, which can justify their twilight habit [3, 16-19].
Discrimination of colors in owls was reported, as well as the light wavelength, which is not important for the behavior of owls [20]. Although the vision of the owls is not as sensitive as previously thought, it still has advantages over the diurnal species at low levels of luminosity [21]. The hearing of Owls is essential for their predatory activity, serving to detect and hunt their prey [22, 23] their vision is intended to determine the spatial orientation, distance and speed fly [24].
The cross-sectional observational study of the species in question was chosen due to the existence of previous morphological and electrophysiological documentation [1], which will be supplemented by the electrophysiological data obtained by applying the diffuse Flash ERG in vivo.
Objective
Presenting the electroretinographic record of Athene cunicularia, identifying their components when the retina is stimulated by the white light spectrum in photopic and scotopic conditions, as well as the relationship between the answers found in both responses and their influence on living species. Providing electrophysiological visual stimulation data of retinal species, encouraging the Biological Sciences with substrate for future research and clinical evaluations.
Materials and Methods
Animals
We studied seven species of owls Athene cunicularia, of both genders, provided by the Brazilian Institute of Environment and Renewable Natural Resources (IBAMA). The animals weighed between 180 - 205 g, and height between 21.8 - 26 cm.
The research protocol complies with the requirements of the Ethics Committee of Faculty of Medicine of Health Sciences Center in Federal University of Rio de Janeiro (protocol number 14/2004) and IBAMA (protocol number 02022.000518/2005- 33).
Methodology of Electroretinography Exam
In order to accomplish the study, we considered as inclusion criteria:
- Animals of both genders;
- Absence of general and ophthalmic anatomical changes;
- Weight within the required standards in the literature; and
- No vitreoretinal pathologies.
The exclusion factors
- Failure to comply with, at least, one of the items of the inclusion criteria.
Following the study protocol, the animals were sedated with ketamine sulfate (20 mg / kg) intramuscular; mydriasis was obtained with topical 1% tropicamide, in a total of three drops per eye. After mydriasis, direct ophthalmoscope was performed on both eyes to exclude vitreoretinal opacities and then topical corneal anesthesia with 0.4% oxybuprocaine hydrochloride at a dose of three drops per eye with thirty second intervals between each drop.
Following the procedures of sedation and anesthesia, three electrodes needle-shaped 13 x 0.45 mm were used for examination. One of the electrodes was introduced into the corneal stroma, causing a self-sealing injury, without the need for sutures or bandages; the other two were inserted into the subcutaneous, one superiorly and between the animal eyes (ground electrode) and the other (reference electrode) in the supraorbital region of the eye to be examined. The electrodes were placed one minute prior to the light stimulus.
Among the fourteen eyes stimulated, seven were destined to photopic response and seven to scotopic response, the choice being made randomly, so that the photopic documentation was obtained of the right eyes and the scotopic documentation of the left eyes.
The electroretinography device used was Neuropack II®, of the company Nihon Koden®. The flash lamp used in all tests, either scotopic or photopic stimuli, had intensity of 20 Joules being performed with stimulator located 30 cm from the animal.
The parameters used for electroretinogram record followed the protocol established by the International Society for Clinical Electrophysiology of Vision (ISCEV) 2004, except for the use of summit Ganzfield (full field), unavailable in the Ophthalmology Department of the Federal University of Rio de Janeiro. The electrodes used were the same and had the same impedance. The duration of the light stimulus was <5 ms. The time to adapt to light to stimulate in photopic environment was 10 minutes and the dark adaptation to stimulus in scotopic environment was 20 minutes [25]. Registered stimuli contemplated white electromagnetic spectrum, visible to man.
Analysis of results
The type of study used was cross-sectional observational analysis, demonstrating the electroretinogram of Athene cunicularia in photopic and scotopic response.
For the evaluation of the data obtained were considered the following conventions:
- -a-wave amplitude: distance between the most negative point of the a-wave and the level of the beginning of the electroretinogram. Unit in microVolts;
- -b-wave amplitude: distance between the most positive point of b-wave and the most negative level of the electroretinogram. Unit in microVolts;
- -b-wave time culmination: distance between the beginning of the stroke and the most positive point of the b- wave. Unit in milliseconds;
- -b-wave latency: distance between the beginning of the stroke and the most negative point of b-wave. Unit in milliseconds.
With reference to the light stimulus studied, we obtained the mean and standard deviation of the above parameters. Due to the small sample used, the statistical evaluation was performed by Student’s t-test two-tailed paired, compared to the same variable in both environments. We determined the error rate (α) of the applied test in order to validate the comparison made. The values of “p” were evaluated for each comparison (photopic or scotopic) and its specific variable.
Results
The electroretinography results of Athene cunicularia were obtained in both photopic and scotopic responses, by being observed all variables proposed for the analysis. They were registered as uneventful strokes in both environments such as under the protocol. They were identified and recorded in the electroretinogram components: a-wave amplitude, b-wave amplitude, the completion time and latency of b-wave.
The t-Student two-tailed paired test was carried out from the same spectrum of light (white), and the same variable in both stimuli, respecting null hypothesis the equality between stimulated conditions, checking that, with an error margin of 1%, there was no rejection of the null hypothesis for all evaluated variables.
The verification of the p-value and t-value, we obtained for the variables, respectively:
- - a-wave amplitude - 0.02 and 2.94;
- -b-wave amplitude - 0.12 and 1.76;
- - b-wave time culmination - 0.07 and 2.12;
- - b-wave latency - 0.74 and 0.34
Discussion
Under the histological appearance of the Athene cunicularia retina, although not object of this study, there are reports of a quite similar structure of vertebrates in general, except for a few peculiarities, such as the presence of rod cells in the macular region and the presence of two foveae [5]. Different histological structures have been described among species of different owls, and probably related to the habit of life of each species [3,26].
The full field ERG expresses the complex electrical response of all retinal cells when stimulated with light [27]. The layout generated by the electrical response is recorded and generates components that can be evaluated separately. Recorded by electrodes, each component represents the activity of various cells of a certain group, and they respond synchronously to the light stimulus. Thus, the ERG record reveals the electrical activity of different cell classes, allowing us to evaluate the retinal functionality.
A comparative study of the b-wave electroretinogram performed under different color spectra has shown, in the studied species, that there is no distinction between the orange color spectrum, yellow and white when comparing the retinal electrical responses in photopic or scotopic stimuli [1]. However, for a better understanding of the electrical activity of the retina it is essential to know the details of the a- and b-waves, which is why the authors chose to study with more details the a-wave and b-wave components of the ERG of the species concerned.
From a clinical point of view, the evaluation of electroretinogram takes place under white light stimulation, which is why the study of the white light was chosen as a reference. The anesthetic ketamine sulfate was used due to its wide application in the veterinary environment and for its inert action on the waves of the electroretinogram, not interfering with the generation of retinal potentials.
It was decided to hold the stimulus under photopic and scotopic stimuli in different eyes, in order to avoid retinal stress in the stimulated eye and a possible corneal edema called out by placing the active electrode on the cornea, which did not occur. The protocol of ISCEV was followed uneventfully, leaving, however, increasingly evident the need for modern electrophysiological equipment, as the summit Ganzfield, which undoubtedly would reduce the loss of light stimulus and significantly increase the fidelity data obtained.
As for the morphological traces, we observed that they are similar to those of the humans, with a- and b-waves, as well as oscillatory potentials. The evaluation of the amplitude of a-and b-waves, as well as the completion time and latency of b-wave were studied because they are relevant measures in the literature and intended to document those components to future standardization of the design of the studied species.
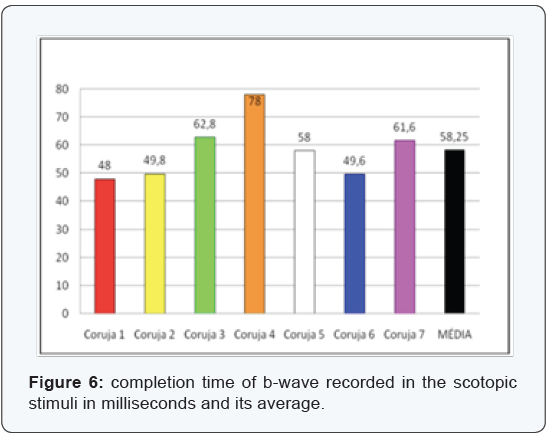
The most clinically important components are the b-wave amplitude and b-wave completion time (Figure 3-6), since the measures relating to a-wave and the b-wave latency are hard to measure and therefore at higher risk of measurement error. In the sample assessed we can confirm the difficulties encountered in the daily clinic actually being the measures of lesser value and most difficult assessment. The latency of the a-wave, for having extremely small amount, about 5 ms, has not been evaluated.
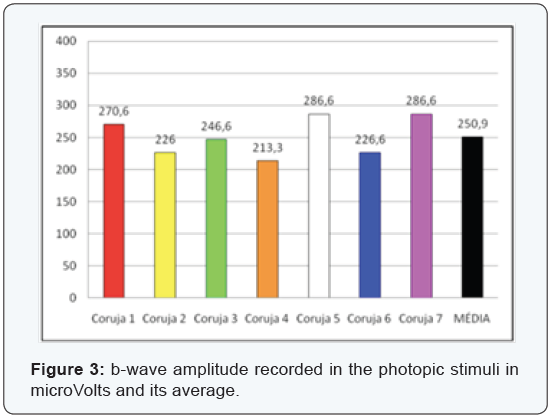
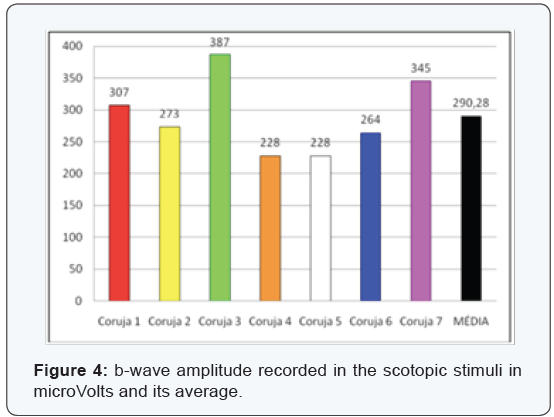
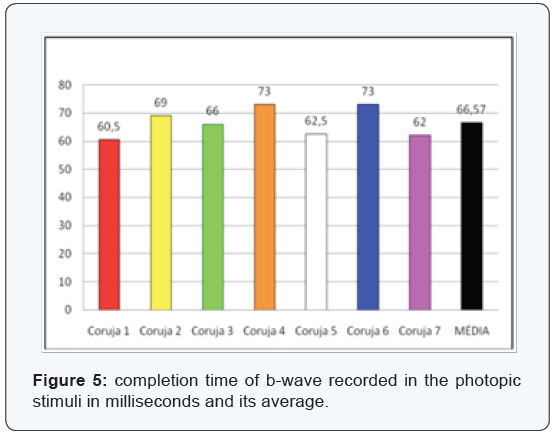
We used the t-Student two-tailed paired test due to the small sample. The data evaluated in the species concerned are unedited from a statistical point, and there are no reports in the literature that refute or ratify why the determination of the “p” value should not exclude data with significance under 95%.
Under the classical evaluation of Granit [28] we could classify the Athene cunicularia retina as a type E, that is, an excitatory type, with a clear PII component, or better, a b-wave. This classification attributed to the retina of the excitatory type a greater activity of the rods; however, now we know that the b-wave is more related to bipolar, horizontal and Müller cells [29-34]. Oscillatory potentials were observed, but were not the object of this study.
The data from the study reflect the reality of the studied species group, and documented all identified values.
The idea that we had in relation to the owls that they have the ability to see either with light or in the dark, may not be confirmed in other species and should continue to be investigated. Their predatory activity and their life habit are due, no doubt, among other characteristics, to the capacity of perception of the light spectrum similarly in both photopic and scotopic stimuli.
The idea that we had in relation to the owls that have ability to see the light and the dark, cannot be confirmed in other species and should continue to be investigated. Their predatory activity and its life habit are due, no doubt, among other characteristics, to the capacity of perception of the light spectrum similarly in both photopic and scotopic stimuli.
Conclusion
The comparative study of the retina in various vertebrate classes has revealed important evolutionary aspects. The Structural differences of the retina as a whole, as well as of the cell types composing it allow us to try to correlate their morphology with the way of life of each animal [3].
The electroretinographic trace of Athene cunicularia under white light stimulus of the white spectrum showed the a- and b-waves, registering all variables proposed for study. From the evaluated data, we can conclude that the retina of the species studied should be classified as of an excitatory type, due to the predominance of the positive component in the ERG.
When comparing the same variable (amplitude of the a- and b-waves and completion time and latency of the b-wave) in both photopic and scotopic stimuli, we observed that retinal electrical response found is identical, confirming the hypothesis that the retina of the studied species responds similarly to white light in both stimuli. This peculiarity is responsible, among other features, for the excellent twilight visual ability of the species, and therefore, directly involved in their life habit. In the biological sciences research the electroretinographic registration of Athene cunicularia may then be a parameter for detailed studies of the physiology of birds and for a better understanding of visual perception as well as the study of eye diseases of the species.
Authors’ Contributions
MP initiated, planned and wrote the manuscript. LR participates in drafting the article and revising it critically for important intellectual content, as well as the analysis and interpretation of data. EF participated in its design and coordination. AM performed the statistical analysis and helped to draft the manuscript, as well as give final approval of the version to be submitted and any revised version. All authors read and approved the final manuscript.
Acknowledgements
The authors thank Carlos Bastos for assistance in all electrophysiological tests, being fundamental in this study; and Fernanda Ramos, for her invaluable contribution in the statistical support. This material is based on work supported by the CEPAL under grant 14/2004 and IBAMA under grant 02022.000518/2005-33. Any opinions, findings and conclusions or recommendations expressed in this article are those of the authors and do not necessarily reflect those of the CEPAL or IBAMA.
References
- Rodrigues MP (2005) Electroretinographic aspects of the Burrowing Owl – Athene cunicularia. Rio de Janeiro,98.
- Lewis DP (2008) Owl Eyes and Vision. The owl pages.
- Sales FC (1990) Morfologia dos Fotorreceptores da Coruja Buraqueira (Speotyto cunicularia). Dissertation - Master’s degree in medicine. Universidade Federal de São Paulo, São Paulo, p.106.
- Oehme H (1961) Vergleichend - histologische Untersuchungen an der Retina von Eulen. Zool Jb Abt Anat Ontog 79: 439-478.
- Fite KV (1973) Anatomical and behavioral correlates of visual acuity in the Great Horned Owl. Vision Res 13(2): 219-230.
- Bowmaker JK, Martin GR (1978) Visual pigments and colour vision in a nocturnal bird, Strix aluco (Tawny Owl). Vision Res 18(9): 1125- 1130.
- Galleno A, Baron M, Gayoso M (1975) Organization of the outer plexiform layer of the diurnal and nocturnal bird retina. Vision Res 15: 1027-1028.
- Morris VB (1982) An afoveal area centralis in the chick retina. J Com Neurol 210: 198-209.
- GInzunza OA. Distribucion de las células ganglionares en la retina de “CORAGYPS ATRATUS”. 1986. Dissertation - Master’s degree in medicine. Escola Paulista de Medicina, São Paulo, 1986. 51p.
- Sales FC, Smith RL (1988) Áster-uma formação na retina de aves foveadas. Estudo comparative Rev Bras Ciên Morfol 5: 44-49.
- Inzunza O, Bravo H, Smith RL (1989) Foveal regions of birds retinas correlate with the aster of the inner nuclear layer. Anat Rec 223: 342- 346.
- TWalls GL (1937) Significance of the foveal depression. Arch Ophthalmol 18(6): 912-919.
- Pumphrey RJ. The sense organs of birds. Ibis, n. 90, p. 171-199,1948a.
- Pumphrey RJ (1948) The theory of the fovea. J Exp Biol 25: 299-312.
- Pumphrey RJ (1961) Sensory Organs: Vision. In: Marshall AJ. Biology and comparative physiology of birds. Academic Press, New York, USA, p. 55-68.
- Dice LR (1945): Minimum intensities of illumination under which owls can find dead prey by sight. Am Nat. 79: 385-416.
- Sillman AJ (1969) The visual pigments of several species of birds. Vision Res 9(9): 1063-1077.
- PSillman AJ (1973) Avian Vision. In: Farner DS & King JR. Avian biology. Academic Press, New York, USA, pp. 349-387.
- Bravo H, Pettigrew JD (1981) The distribution of neurons projecting from the retina and visual cortex to the thalamus and tectum opticum of the Barn Owl, Tyto alba, and the Burrowing owl, Speotyto cunicularia. J Comp Neurol 199(3): 419-441.
- Martin GR (1974) Color vision in the tawny owl (Strix aluco). J Comp Physiol Psycol 86(1): 133-141.
- Martin GR (1977) Absolute visual threshold and scotopic spectral sensitivity in the tawny owl strix aluco. Nature 268: 636-638.
- Konishi M (1973) How the owl tracks its prey. Am Sci 61: 414-424.
- Martin GR (1986) Sensory capacities and the nocturnal habit of owls (Strigifomes). Ibis 128: 266-277.
- Erkert HG (1969) Die Bedeutung des Lichtsinnes für aktivität und Raumorientatierung der Schleiereule (Tyto alba guttata Brehm). Z Vergl Physiol 64: 37-70.
- Marmor MF, Holder GE, Seeliger MW, Yamamoto S (2004) Standard for clinical electroretinography (2004 update). Doc Ophthalmol 108(2): 107-114.
- Roze M, Luccciani A, Auphan ML (1990) oeil des rapaces – Approche électrorétinographique et histologique. Ophthalmologie 4: 66-68.
- Dantas AM (1977) O Eletrorretinograma e os Potenciais Oscilatórios – Contribuição Experimental e Clínica. Tese - Faculdade de Medicina da Universidade Federal Fluminense - Universidade Federal Fuminense, Niterói-RJ, p: 229.
- Granit R (1933) The components of the retinal action potential in mammals and their relation to the discharge in the optic nerve. J Physiol 77(3): 207-239.
- Brown KT, Murakami M (1964) A new receptor potential of the monkey retina with no detectable latency. Nature 201: 626-628.
- Pak WL, Cone RA (1965) Isolation and identification of the initial peak of the early receptor potential. Nature 204: 836-838.
- Goldstein EB, Berson EL (1970) Rod and cone contributions to the human early receptor potential. Vis Res 10(3): 207-218.
- Sieving PA, Fishman GA (1982) Rod contribution to the human early receptor potencial (EPR) estimated from monochromat’s data. Doc Ophthalmol Proc ser 31: 95-102.
- Hood DC, Brich DG (1996) b-wave of the scotopic (rod) electroretinogram as a measure of the activity of humn on-bipolar cells. J Opt Soc Am 13(3): 623-633.
- Bush RA, Sieving PA (1994) A proximal retinal component in the primate photopic ERG a- wave. Invest Ophtalmol Vis Sci 35(2): 635- 645.







