Unexpected Adverse Effect of Clofarabin Plus Cytosine Arabinoside: Bronze Hyperpigmentation
Aysun Şenturk Yikilmaz1, Sema Akinci2, Sule Mine Bakanay1, Kamile Silay3*, Imdat Dilek1
1Department of Hematology, YUdirim Beyazit University, Turkey
2Department of Hematology, Ataturk Training and Research Hospital, Ankara, Turkey
3Department of Geriatrics, Yildirim Beyazit University, Turkey
Submission: December 15, 2017; Published: March 20, 2018
*Corresponding author: Kamile Silay, Department of Geriatrics, Yildirim Beyazit University, Turkey, Email: kamilesilay@hotmail.com
How to cite this article: Aysun p Y, Sema A, Sule M B, Kamile S, Imdat D. Unexpected Adverse Effect of Clofarabyn Plus Cytosyne Arabynosyde: Bronze Hyperpygmentatyon. JOJ Nurse Health Care. 2018; 6(5): 555698. DOI: 10.19080/JOJNHC.2018.06.555698
Abstract
Objective: Rare unexpected adverse effect
Background: Skin toxicity in acute myeloid leukemia(AML) is a condition that with a lot of chemotherapy agents. Clofarabine is a second- generation purine nucleoside analogs used in the treatment of AML. Diarrhea, liver toxicity, skin toxicity, hand-foot syndrome, mucous membrane involvement, renal failure, bone marrow suppression may be listed as a side effect of Clofarabine.
Case Report: In this report, we describe the case of a 74-year-old male with refractory acute myeloid leukemia with skin toxicity as bronze hyperpigmentation, diarrhea, hyperbilirubinemia and liver toxicity associated with clofarabine treatment.
Conclusions: The side effects of clofarabine in the second course may be more aggressive than the first course. The bronze hyperpigmentation with clofarabine should also be meant to keep in mind.
Keywords: Clofarabine; Bronze hyperpigmentation; Acute myeloid leukemia
Abbreviations: AML: Acute Myeloid Leukemia; HSCT:Hematopoietic Stem Cell Transplantation
Background
Skin toxicity in acute myeloid leukemia(AML) is a condition that with a lot of chemotherapy agents [1]. We report a patient with relapsed AML, who developed generalized bronze pigmentation of skin after a second cycle of clofarabine plus high dose Ara-C.
Case Report
In 2013, a 74-year-old male patient was evaluated because of fatigue and bicytopenia and was diagnosed as AML M4. The patient received the 7+3 induction therapy with Ara-C and idarubicine followed by 3 cycles of high dose Ara-C consolidation. Since the patient had a good performance status and no comorbid conditions, allogeneic hematopoietic stem cell transplantation (HSCT) with reduced intensity conditioning was planned. However, a search for an HLA-matched family or an unrelated donor failed to find a suitable donor. The patient relapsed 11 months after the initial diagnosis. Salvage chemotherapy with clofarabine 40 mg/ m2/day and Ara-C 2g/m2/day for five days was administered. Diarrhae began on the second day of the first cycle and stopped on the eleventh day, electrolyte replacement was not required. Transient hyperbilirubinemia with a total billuribin of 5.9 mg/ dl and mild increase in transaminases were observed on the fifth day. The patient became febrile on the fourth day of chemotherapy and was treated with appropriate antibiotics. The patient's blood counts recovered on the twenty-fourth day and a control bone marrow biopsy before discharge was in hematologic remission. After six weeks, the patient was rehospitalized for a second cycle of clofarabine and Ara-C. Severe diarrhea which required electrolyte replacement and a moderate to severe increase in transaminases (AST/ALT: 425/ 625 U/I) were observed on the third and the fourth days of the chemotherapy, respectively. On the seventh day of the treatment cycle, the patient developed bronze pigmentation on the whole body surface. The patient had an extended febrile neutropenic period and antifungal therapy was added due to suspected aspergillus infection on thorax CT. However, the patient died of sepsis before recovery of blood counts.
Discussion
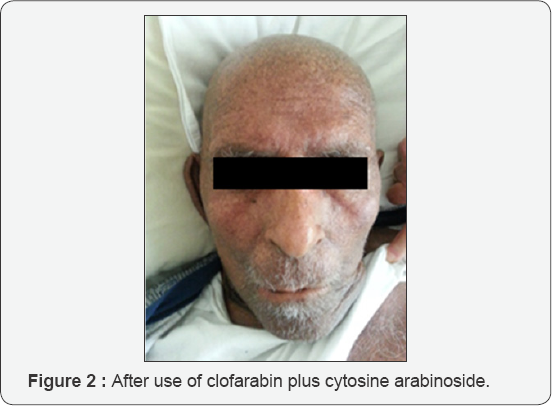
Survival of elderly patients with relapsed/refractory AML is low. Allogeneic HSCT with reduced intensity conditioning regimens has increasingly been performed in fit -elderly population. However, not all patients can receive such a curative approach either due to comorbid conditions or lack of an available donor. Clofarabine in combination with Ara-C has been shown to be effective in elderly patients both as induction and salvage regimen [2]. Clofarabine is a second generation purine analog designed to combine the favorable properties of fludarabine and 2-chlorodeoxyadenosine. Thus, when administered before Ara-C, it might enhance intracellular conversion of Ara-C to its active metabolite Ara-C TP providing beter cell killing [3]. In the only randomized phase III study, clofarabine plus Ara-C was compared with Ara-C alone in patients (≥ 55 years) with relapsed/refractory AML and better ORR and EFS with clofarabine plus Ara-C was reported. This protocol although did not prolong the OS compared with the Ara-C arm, allowed more patients to proceed to HSCT The main grade 3-4 toxicities were myelosuppression, febrile neutropenia, pneumonia and increased transaminases. They also reported skin toxicity mainly as non-specific skin rash or hand- foot syndrome [4]. Ara-C dose in most studies is 1g/m2/day. In our patient, we administered Ara-C 2g/m2/day depending on a report by Tse et al, who demonstrated higher CR rate with Ara-C 2g/m2/ day versus Ara-C 1g/m2/day [5]. Most common adverse events observed with clofarabine containing regimens are diarrhea, liver toxicity, skin rash, hand-footsyndrome, mucous membrane involvement, renal failure, bone marrow suppression [6] (Figures 1 & 2).

Conclusion
To our knowledge, bronze pigmentation of skin after clofarabine plus Ara-C combination has not been reported so far. Besides, increased mortality has been observed especially due to grade 4 myelosuppression and propensity to sepsis. This should be taken into account when administering clofarabine plus Ara-C to elderly AML population. The efficacy and safety of sequential administration of clofarabine plus Ara-C chemotherapy requires further investigation.
References
- Zhang B, Bolognia J, Marks P, Podoltsev N (2014) Enhanced skin toxicity associated with the combination of clofarabine plus cytarabine for the treatment of acute leukemia. Cancer Chemother Pharmacol 74(2): 303-307.
- Fozza C (2015) The role of Clofarabine in the treatment of adults with acute myeloid leukemia. Crit Rev Oncol Hematol 93(3): 237-245.
- Gandhi V, Estey E, Keating MJ, Plunkett W (1993) Fludarabine potentiates metabolism of cytarabine in patients with acute myelogenous leukemia during therapy. J ClinOncol 11(1): 116-124.
- Faderl S, Wetzler M, Rizzieri D, Schiller G, Jagasia M, et al. (2012) Clofarabine plus cytarabine compared with cytarabine alone in older patients with relapsed or refractory acute myelogenous leukemia: results from the CLASSIC I Trial. J ClinOncol 30(20): 2492-2499.
- Tse E, Leung AY, Sim J, Lee HK, Liu HS, et al. (2011) Clofarabine and high-dose cytosine arabinoside in the treatment of refractory or relapsed acute myeloid leukaemia. Ann Hematol 90(11): 1277-1281.
- Faderl S, Ferrajoli A, Wierda W, Huang X, Verstovsek S, et al. (2008) Clofarabine Combinations as Acute Myeloid Leukemia Salvage Therapy. Cancer 113(8): 2090-2096.






























