Microbiological Assessment and Storage Quality of Expressed Breast Milk
Ifeanyi OC Obiajuru1*, Chidinma A Ikpeama2 and Jacinta C Elo-Ilo3
1Department of Microbiology, Imo State University, Nigeria
2Department of Animal and Environmental Biology, Imo State University, Nigeria
3Department of Paediatrics, Nnamdi Azikiwe University, Nigeria
Submission: April 15, 2017; Published: July 14, 2017
*Corresponding author: Ifeanyi OC Obiajuru, Department of Microbiology, Faculty of Medicine, Imo State University, Nigeria, Email: drifeanyi_oc@yahoo,co.uk
How to cite this article: Ifeanyi OC O, Chidinma A I, Jacinta C E. Microbiological Assessment and Storage Quality of Expressed Breast Milk. JOJ Nurse Health Care. 2017; 2(5): 555597. DOI: 10.19080/JOJNHC.2017.02.555597
Abstract
The microbiological and storage quality of expressed human breast milk was studied between July and December, 2016. One hundred and twenty working class lactating mothers and thirty lactating mothers visiting Imo State University teaching Hospital Orlu for various health challenges were recruited for the study. They were requested to express 60ml of their breast milk into sterile containers. The milk sample collected from each mother was distributed 10ml into each of 3 sterile containers. One set was heated at 100 °C for 1hour in a water bath, 1 set was stored in a refrigerator at- 4 °C for 5 days and 1 set was stored on the bench at ambient temperature without any treatment. 0.1ml of each sample was inoculated on laboratory culture media before commencement of storage and 2hours, 6hours, 12 hours, 24 hours and 5days post storage. Eight genera of bacteria: Stapylococcus aureus, Streptococcus viridians, Diphtheroides, Escherichia coli, Klebsiella species, Lactobacillus species, Pseudomonas species and Salmonella species, were isolated from expressed human breast milk samples. The most prevalent bacterium in the milk samples was Staphylococcus epidernidis, followed by Escherichia coli. The least prevalent bacteria were Pseudomonas aeruginosa, Salmonella species and Diphtheroides. No bacterium was isolated from milk samples heated at 100 °C and stored in a refrigerator. The total heterotrophic bacterial counts of the milk samples collected from healthy working mothers ranged from 3.2x103 to 8.2x103cfu/ml, while that of health challenged mothers ranged from 4.3x103 to 1.6x104cfu/ml. As shown, the bacterial counts of the samples. Out of 30 samples collected from health-challenged mothers, 9 (30%) had total heterotrophic bacteria count ranging from 1.2x104 to 1.6x104cfu/ml, 21 (70%) had total bacteria count ranging from 4.3x103 to 8.6x103cfu/ml. Analysis of the data using chi square showed significant difference (p<0.05) in the total heterotrophic bacterial count of breast milk between healthy working mothers and health challenged mother.
Keywords: Microbiological; Storage; Quality; Expressed-breast milk
Introduction
Man's basic needs remain food, clothing and shelter. The nutritional needs of children are quite different from that of adults both in quantity and quality [1]. Although a number of dietary substances may be available for children, the new born babies depend largely on milk based diet. Roy & Lescop [2] stated that human breast milk remains the best source of nutrition for infants for several reasons. It contains immunoglobulins and active leucocytes that enhance resistance to infections [3]. Doctors and primary healthcare givers advocate largely for exclusive breast-feeding, at least for the first 6 months of life. Paediatricians claim that the decline in breast-feeding has led to an increase in infant mortality resulting from microbial infections. The presence of 2 mirror proteins: lactoferrin and lactoperoxidase in human breast milk is associated with antibacterial properties [4]. Citing a UNICEF report of 1983, Sasson Albert [1] stated that bottle-fed children are 3 to 6 times more likely to die prematurely in developing countries than breast-fed children. This is because when new born babies are breast-fed the dominant flora of their digestive tract at the end of first week of life consist mainly of Bifidobacterium; the coli bacteria and the Streptococcus are usually smaller in number, as are anaerobic bacteria of the Bacteroides species.
Mothers are not always disposed to breastfeed their babies adequately. Many mothers are working in public service, industries and private sector. This made it difficult for them to adequately breast feed their babies. WHO [5] reported that the breastfeeding working mother is often forced to give the baby breast milk substitute or supplements while she is away from home. Some medical and social factors make it difficult for many mothers to fully breastfeed their babies. The need to provide to premature babies and other distressed babies made healthcare providers advocate seriously for human breast milk bank. Although this has been in practice in many countries for some years, there is no clear guideline for collection, processing and storage of human breast milk for therapeutic use [3]. In Nigeria, much work has not been done on the microbiological quality of expressed stored human breast milk. Milk is a good medium for the growth and multiplication of many micro-organisms, thereby making it a possible means of transmission of microbial infections when it is not properly collected, processed and stored. Asquith & Harrod [6] stated that milk samples having bacterial counts of 10,000cfu/milliliter are not suitable for consumption. This study was undertaken to determine microbiological quality of expressed human breast milk with particular reference to the common storage conditions used in Nigeria.
Materials and Methods
This study was carried out at Imo State University Teaching Hospital, Orlu, south eastern Nigeria. Ethical permit was obtained from the Ethical Committee of the hospital. One hundred and twenty working class lactating mothers and 30 lactating mothers visiting Imo State University teaching Hospital Orlu for various health challenges were recruited for the study, between July and December, 2016. Their consent and willingness to participate in the study was obtained by signing the consent form on the study questionnaire. They were requested to complete the study questionnaire and express 80-100ml of their breast milk into sterile containers. The completed questionnaires and expressed breast milk samples were taken to the Microbiology laboratory of the hospital for analyses.
Each sample was distributed 10ml into each of 3 sterile containers and labeled properly. One was heated at 100 °C for 1hour in a water bath and stored in a refrigerator, 1 was stored in a refrigerator at -4 °C and 1 was stored on the bench at ambient temperature. They were allowed to stay for 5days. 0.1ml of each sample was inoculated on laboratory culture media before commencement of storage and 6 hours, 12 hours, 24 hours and 5 days post storage. Total heterotrophic bacterial counts of the samples were determined using serial dilution of each sample and inoculating 0.1ml dilution on Nutrient agar plate as in Obiajuru & Ozumba [7].
The bacterial isolates were subjected to antibiotic susceptibility tests using Mueller Hinton agar and commercially prepared antibiotic discs of Gentamicin 30μg, Levofloxacin, Ampicclox, Streptomycin, Ciprofloxacin 30μg, Augumentin, Ceftriazone 30μg, Ceftazidime 30|ig, Azetreonam 30μg and Amoxycillin-Clavulanic acid 30μg (Oxoid, UK.) as in Cheesbrough [8], Obiajuru & Ozumba [7]. The zones of growth inhibitions exhibited by the different antibiotics were measured using transparent metric rule
Results
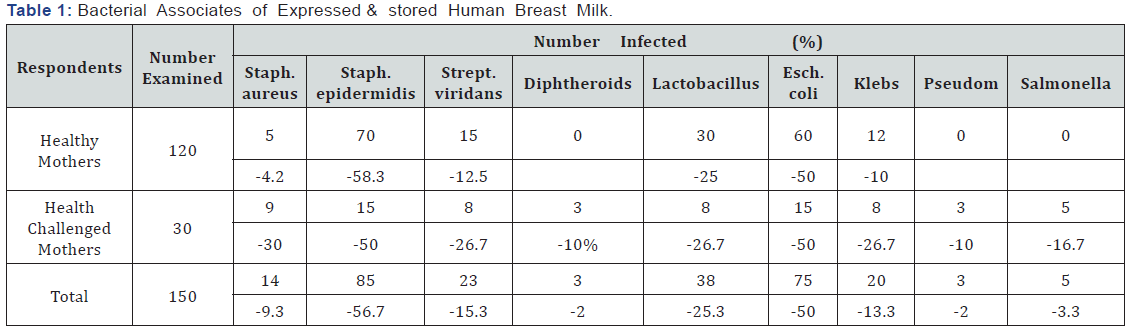
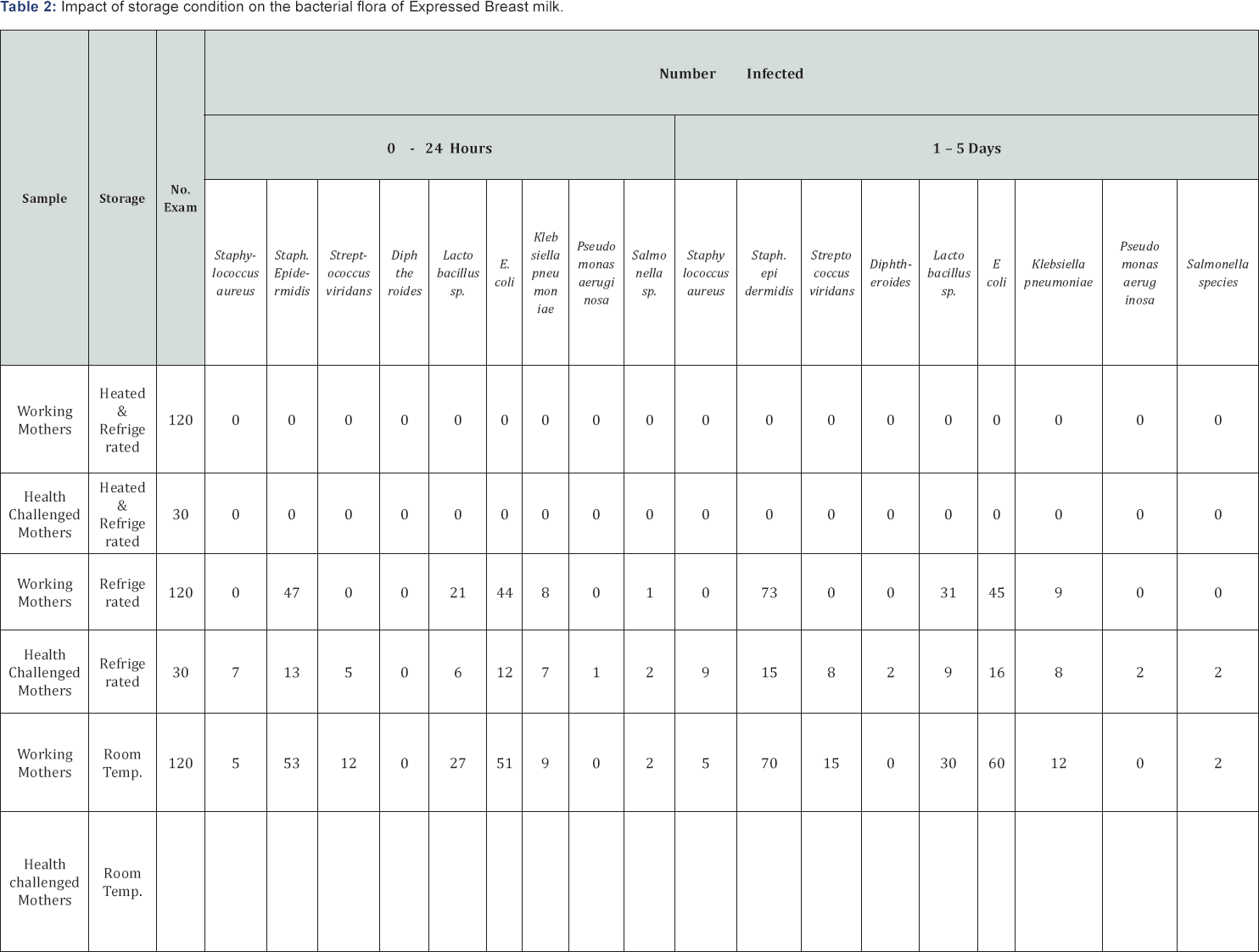
Eight genera of bacteria: Stapylococcus aureus, Streptococcus viridians, Diphtheroides, Escherichia coli, Klebsiella species, Lactobacillus species, Pseudomonas species and Salmonella species, were isolated from expressed human breast milk samples. Table 1 summarizes the bacterial associates of breast milk samples. As shown, the most prevalent bacterium in the milk samples (56.7%) was Staphylococcus epidernidis, followed by Escherichia coli (50%). The least prevalent bacteria (2.0%) were Pseudomonas aeruginosa, Salmonella species and Diphtheroides. Table 2 summarizes effect of storage conditions on the bacterial flora of expressed breast milk. As shown, no bacterium was isolated from milk samples heated at 100OC and stored in a refrigerator. Pseudomonas aeruginosa, Diphtheroides and Salmonella species were isolated from breast milk of health challenged mothers only. The total heterotrophic bacterial counts of the milk samples collected from apparently healthy working mothers ranged from 3.2x103 to 8.2x103cfu/ml, while that of health challenged mothers ranged from 4.3x103 to 1.6x104cfu/ ml. As shown, the bacterial counts of the samples. Out of 30 samples collected from health -challenged mothers, 9 (30%) had total heterotrophic bacteria count ranging from 1.2x104 to 1.6x104cfu/ml, 21 (70%) had total bacteria count ranging from 4.3x103 to 8.6x103cfu/ml. Analysis of the data using chi square showed significant difference (p<0.05) in the total heterotrophic bacterial count of breast milk between healthy working mothers and health challenged mother. Also there was significant difference (p<0.05) in the bacterial count between breast milk samples stored in the refrigerator and those stored on the bench at room temperature. The bacteria count of samples stored in the refrigerator was lower than those stored at room temperature. Breast milk heated at 100 °C for 1 hour in a water bath and stored in a refrigerator had no bacterial growth. Table 3 summarizes the antibiotic susceptibility pattern of bacterial associates of expressed human breast milk in Orlu. As shown, Diphtheroids exhibited 100% susceptibility to ciprofloxacin, levofloxacin, ceftriaxone and ceftazidime. Pseudomonas aeruginosa and Salmonella species exhibited 100% susceptibility to ceftazidime. Staphylococcus epidermidis exhibited the highest susceptibility (89.4%) to ceftriaxone while Staphylococcus aureus exhibited the highest percentage of susceptibility (85.7%) on ceftazime. Streptococcus viridians, Lactobacillus species and Escherichia coli exhibited the highest percentages (91.3, 92.1 and 93.3 respectively) of susceptibility to levofloxacin. Diphtheroids, Pseudomonas aeruginosa, Klebsiella species and Salmonella species, were resistant to streptomycin, ampiclox, augumentin and erythromycin. Statistical analysis of the data using Chi square showed significant difference (p<0.05) in the susceptibility pattern between the Gram-positive and Gram-negative bacterial isolates.
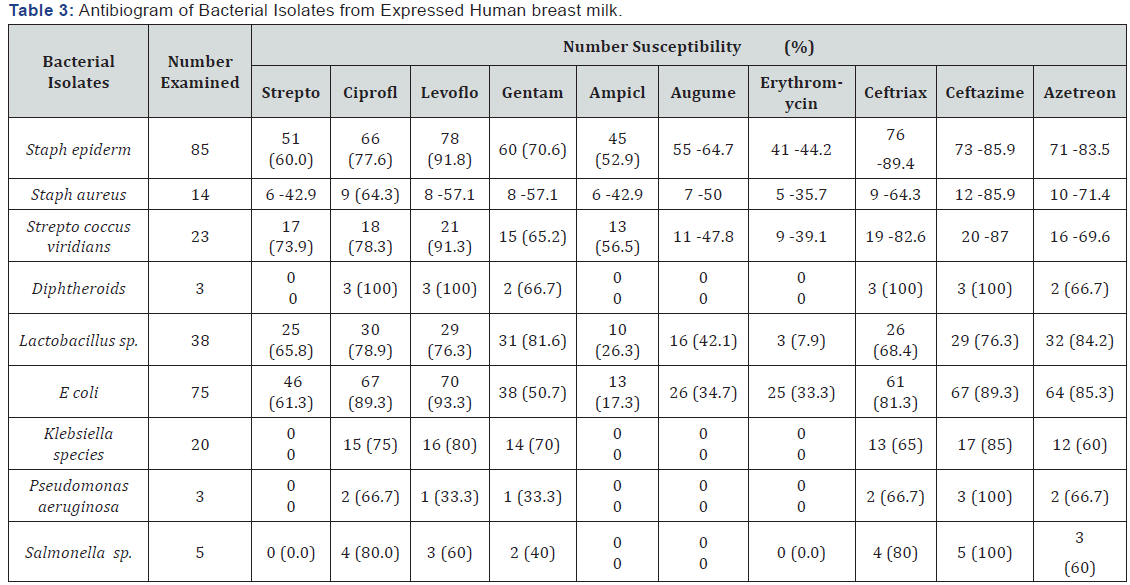
The antibiotic susceptibility pattern of the bacterial isolates showed that the bacteria present in the breast milk are susceptible to conventional antibiotics commonly used in Nigerian clinics. In case any microbial infection arises through the use of banked human breast milk, antibiotic drugs capable of providing treatment and care are readily available.
Discussion
Human breast milk has advantages over infant formula in preventing neonatal sepsis and necrotizing entero-colitis [9-11]. Notwithstanding, human breast milk is a potential source of transmission of microbial infections. The present study isolated bacteria species capable of causing infections in human. Salmonella which was isolated from breast milk of 2 healthy working mothers and 3 health-challenged mothers, is known to be the causative agent of typhoid and paratyphoid fever. Staphylococcus aureus isolated from 5 healthy working mothers and 9 health-challenged mothers, is associated with food poisoning and other human infection. Previous workers have linked human breast with Salmonella infection [12], tuberculosis infection [13], methicillin-resistant Staphylococcus aureus infection [14], Streptoccocal infection [15], and Listeria infection [16].
While appreciating the obvious advantages of human breast milk over infant formula, we recommend that adequate measures should be taken to preserve expressed human breast milk to safeguard the babies who feed on them. Our study showed that when breast milk is heated to 100 °C in a water bath and refrigerated, it could remain free of bacteria and thus safe for upto 5days. Refrigeration of expressed breast milk without sterilization or preservation at room temperature should not be encouraged beyond 24 hours.
The total heterotrophic bacteria count of 141 (%) breast milk samples used in this study were within acceptable count i.e. below1.0x10 cfu/ml [6]. However the bacteria count of 9 (%) obtained from health challenged mothers were well above acceptable standard. This suggests that expressed breast milk banked for therapeutic use should pass through quality control to ensure that they will not constitute possible sources of infecting babies. The source of bacterial contamination of breast milk used in this study was not investigated, however, majority of the isolates were common bacteria associated with human bodies and human activities. Staphylococcus epidermidis is a normal flora of the skin of most humans. Escherichia coli is a common bacteria associated with the human gastrointestinal tract. It is likely that the bacteria isolates were from the mothers whose breast milk were used for the study. WHO [5] reported that the method of collection of breast milk may influence the composition, quality and quantity. Other workers, [17-19] reported the degree of pressure used to express breast milk influences the fat content, etc. Adequate care should therefore be taken when expressing breast milk to be stored in milk bank to reduce the risk of bacterial contamination. Asceptic methods should be ensured during collection and banking of breast milk.
This study has shown that expressed human breast milk can be banked effectively for upto 5 day without compromising its microbiological quality. The decline in breast feeding for various social and medical reasons should be discouraged and improved methods of expression and banking of human breast milk supported to provide adequate nutrition for distressed babies and premature babies in intensive care units. Policy makers in the country should help set standards to guide milk banking.
References
- Sasson A (1990) Feeding Tomorrow's World. UNESCO / CTA. Paris, pp. 805.
- Roy CC, Lescop I (1979) Human milk banking : High rate of interest for a still uncertain credit balance. American Journal of Dis Child 133(3): 255-256.
- Deodhar L, Joshi S (1991) Microbiological study of breast milk with special reference to its storage in milk bank. J Postgrad Med 37(1): 14-16.
- Ghogan (2013) Breast milk contains over 700 bacteria species.
- WHO (1985) The Quantity and Quality of breast milk: Report on the collaborative study on Breast feeding. World Health Organization. Geneva, Switzerland pp.148.
- Asquith MT, Harrod JR (1979) Reduction of bacterial contamination in banked human milk. J Pediatr 95(6): 993-994.
- Obiajuru IO, Ozumba UC (2009) Laboratory Methods for Medical Microbiology and Parasitology. Lifeway Amalgamations, Owerri, Nigeria. pp.183.
- Chesbrough M (2002) Medical Laboratory Manual for Tropical Countries. ELSA with Tropical Health Technology Butterworth Heinemann Ltd Oxford 1: 605- 607.
- Hylander MA, Strobino DM, Dhanireddy R (1998) Human milk feeding and infection among very low birth weight infants. Paediatrics 102(3): E38.
- Hanson LA, Korotkova M (2002) The role of breast feeding in prevention of neonatal infection. Semin Neonatal 7(4): 275-281.
- 11. Chen A, Rogan W J (2004) Breast feeding and the risk of neonatal death in the United States. Pediatrics 113(5): e435- e439.
- Qutaishat SS, Stemper ME, Spencer SK, Borchardt MA, Opitz JC, et al. (2003) Transmission of Salmonella enterica serotype Typhimurin DT104 to infants through mother's breast milk. Pediatrics 111(1): 1442-1446.
- Pronczuk J. Akre j, Moy G, Vallenas C (2002) Global perspectives in breast milk contamination: infectious and toxic hazards. Environ Health Perspect 110: A349-A35.
- Gastelum DT, Dassey D, Mascola, L, Yasuda LM (2005) Transmission of community associated methicillin resistant Staphylococcus aureus from breast milk in neonatal intensive care unit. Pediatr Infect Dis J 24(12): 1122-1124.
- Kotiw M, Zhang GW, Daggard G, Reoss - Levy E, Tapsall JW, et al. (2003) Late onset and recurrent neonatal Group B Streptococcal disease associated with breast milk transmission. Paediatric Dev Path 6(3): 251-256.
- Svabic-Vlahovic M, Pantic D, Pavicic M, Gryner AH (1998) Transmission of Listeriamonocytogenes from mother's milk to her babies and puppies. Lancet 2(8621): 1201.
- Widdows ST, Lowenfed MF (1983) Biochemical Journal 27: 1400.
- Novak FR, Da Silva AV, Hagler AN, Figueiredo AMS (2000) Contamination of expressed human breast milk with an epidemic multi- resistant Staphylococcus aureus clone. J Med Microbiol 49(12): 1109-1117.
- UNICEF (1993) In: Sasson A (1990) Feeding Tomorrow's World. UNESCO / CTA. Paris, pp. 805.






























