Bump, Cyst, Conversion-Chondrosarcoma
Anubha Bajaj*
Department of Histopathology, Panjab University, India
Submission: November 10, 2022; Published: November 22, 2022
*Corresponding author: Anubha Bajaj, Department of Histopathology, Panjab University, India
How to cite this article: Anubha B. Bump, Cyst, Conversion-Chondrosarcoma. JOJ Int Med. 2022; 1(3): 555565. DOI:10.19080/JOJIM.2022.01.555565
Abstract
Chondrosarcoma is a primary, malignant bone tumour characteristically configuring a cartilaginous matrix demonstrating chondrocytes embedded within distinct lacunae. Chondrosarcoma frequently incriminates pelvic bones, femur, or humerus. Chondrosarcoma depicts genomic mutations within IDH1 and IDH2 genes, TP53, aneuploidy and active signalling pathways incorporating RB1, CDKN2A or CDK. Neoplasm exemplifies clinical symptoms such as pain, localized swelling or enlarging tumefaction. Variably and preponderantly cellular chondrosarcoma exhibits significant nuclear atypia, mitotic activity and a lobular or diffuse tumour configuration. An abundant cartilaginous matrix with embedded chondrocytes permeating lacunae or inter-trabecular spaces is observed. Chondrosarcoma requires segregation from chondroblast osteosarcoma, enchondroma, fracture callus or chondromyxoid fibroma. Surgical extermination of neoplasm along with removal of broad perimeter of uninvolved tissue is optimal.
Keywords: Cartilaginous tumour; Chondrocytes; Lacunae; Matrix
Introduction
Chondrosarcoma is a locally aggressive, primary, malignant bone tumour characteristically configuring a cartilaginous matrix with chondrocytes embedded within distinct lacunae. Chondrosarcoma contributes to ~20% of primary, malignant bone tumours and follows osteosarcoma in frequency. Conventional chondrosarcoma is sub-classified into primary, secondary, and periosteal chondrosarcoma. Periosteal chondrosarcoma may be denominated as juxta-cortical chondrosarcoma. Low grade neoplasms may be scripted as low grade, cartilaginous neoplasm. Chondrosarcoma grade I is designated as atypical cartilaginous tumour and commonly incriminates appendicular skeleton. Mesenchymal and clear cell chondrosarcoma may infrequently be observed [1,2].
Primary chondrosarcoma incriminates elderly population or middle-aged individuals whereas secondary and periosteal chondrosarcoma implicates younger subjects. A male predominance is observed. Chondrosarcoma frequently incriminates pelvic bones, femur, and humerus. Additionally, tumefaction is observed within trunk, skull, or facial bones. Small bones of hands and feet are exceptionally involved. Periosteal chondrosarcoma incriminates metaphysis of long bones as distal femur or humerus [1,2]. Conventional chondrosarcoma frequently emerges within the larynx.
Around ~50% chondrosarcomas depict genomic mutations within IDH1 and IDH2 genes. Tumours of advanced grade demonstrate aneuploidy. Besides, chromosomal mutations within TP53 and associated active signalling pathways incorporating RB1, CDKN2A or CDK may emerge within high grade chondrosarcomas. Primary chondrosarcoma is a neoplasm of obscure aetiology and appears devoid of pertinent benign precursor lesions [1,2]. Secondary chondrosarcoma may arise due to malignant metamorphosis of benign precursors. Central secondary chondrosarcoma emerges within pre-existing enchondroma whereas peripheral secondary chondrosarcoma occurs within pre-existing cartilaginous cap of an osteochondroma [1,2].
Enhanced possible emergence of secondary chondrosarcoma is discerned with Ollier’s disease and Maffucci syndrome. Periosteal chondrosarcoma arises upon the surface of bone in association with bony periosteum [1,2]. Chondrosarcoma exemplifies clinical symptoms such as pain, localized swelling, and enlarging tumefaction. Neoplasms incriminating skull base may demonstrate neurological symptoms. Enchondroma or osteochondroma may undergo malignant metamorphosis indicated by altered tumour magnitude or cogent clinical symptoms [1,2].
Grossly, neoplastic hyaline cartilage appears lobular. A cut surface demonstrates a grey/tan appearance with and foci of myxoid or mucoid substance. Focal mineralization enunciates chalky deposits of calcium. Erosion of bony cortex and neoplastic infiltration into circumscribing soft tissue ensues [1,2]. Secondary peripheral chondrosarcoma delineates a thick cartilaginous cap of magnitude ~2 centimeters incorporated with cystic cavities. Periosteal chondrosarcoma manifests as an enlarged, lobulated tumefaction adhering to surface of subjacent bone [1,2].
The frozen section exemplifies nodules of hyaline cartilage representing variable atypia. Low grade tumefaction can be nomenclated as ‘low grade cartilaginous neoplasm’ [1,2]. Upon cytological examination, an abundant extracellular matrix appears admixed with definitive lacunae permeated with binucleated or multinucleated chondrocytes. Atypical cartilaginous tumour or grade I chondrosarcoma morphologically simulates an enchondroma [1,2]. Grade II or III chondrosarcoma is cellular and composed of cells with significant cytological and nuclear atypia intermingled within abundant myxoid matrix [1,2].
In contrast to primary chondrosarcoma, metastatic chondrosarcoma is adequately discerned upon cytological examination [1,2]. Upon microscopy, tumefaction is variably and preponderantly cellular with significant nuclear atypia and mitotic activity. Contingent to grade, neoplasm exhibits lobular or diffuse pattern of tumour configuration. An abundant cartilaginous matrix demonstrates chondrocytes embedded within lacunae with permeation of inter-trabecular spaces.
Foci of myxoid alterations and tumour necrosis are observed. Chondroid matrix may demonstrate liquefaction. The low grade, secondary peripheral chondrosarcoma may configure nodules and cystic cavities [1,2]. Periosteal chondrosarcoma commonly manifests as low grade, grade I or grade II neoplasm confined to extraneous bony surface. Foci of infiltration into bony cortex or circumscribing soft tissue and tumour magnitude > 5 centimeters favors a malignant tumefaction, in contrast to periosteal chondroma [1,2] (Figure 1 & 2).
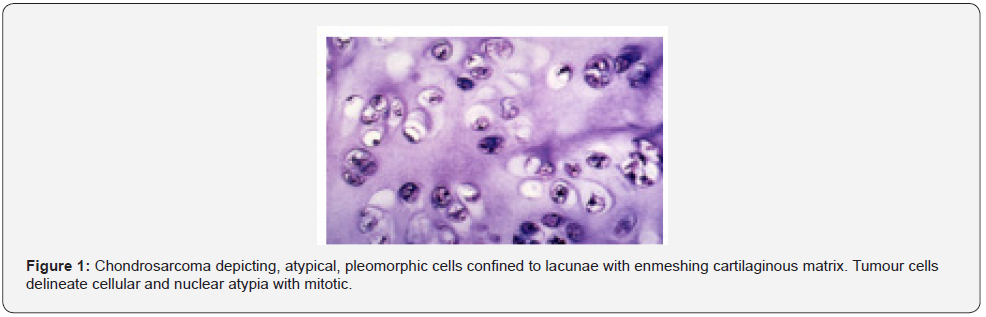
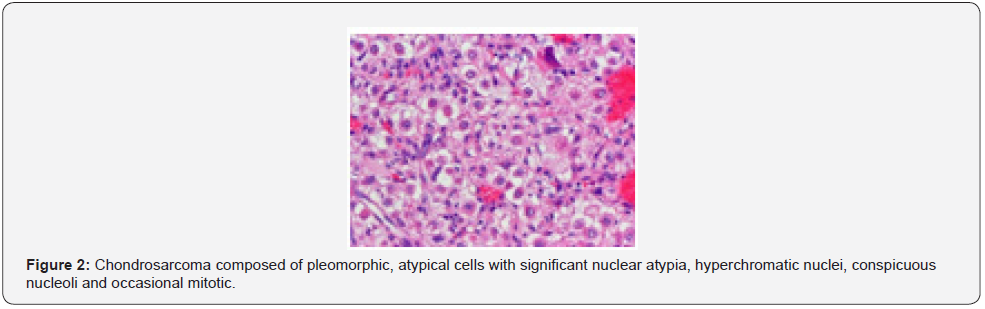
Histological Behaviour of Chondrosarcoma is Contingent to Tumour Grade and is Designated as
a) Grade I composed of low grade, locally aggressive neoplasm with a nodular pattern of tumour evolution, denominated as atypical cartilaginous tumour. Grade I lesion simulate normal cartilage or benign enchondroma. Distinction between benign and malignant tumefaction is contingent to detection of ‘chondrosarcoma permeation pattern’ with tumour infiltration through bone marrow cavity. Grade I chondrosarcoma is minimally or moderately cellular wherein neoplastic cells demonstrate plump, uniform, hyperchromatic nuclei. Bi-nucleate nuclei are occasionally discerned.
b) Grade II exemplifies moderately cellular neoplasms with diffuse pattern of tumour progression. Nuclear atypia with hyperchromatic nuclei, altered nuclear magnitude and mitotic activity is discernible.
c) Grade III enunciates neoplasms of enhanced cellularity. Tumour cells are preponderantly atypical, pleomorphic and depict frequent mitotic activity. Neoplastic cells confined to periphery of tumour lobules appear spindle-shaped and minimally differentiated.
d) Grade IV manifests dedifferentiated chondrosarcoma (~10%) morphologically demonstrating a high grade, pleomorphic, spindle-shaped tumefaction devoid of significant cartilaginous matrix and associated with an adverse prognosis [2,3].
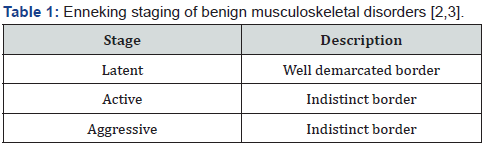
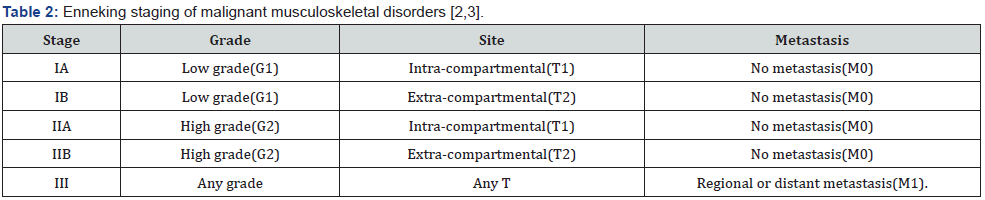
Chondrosarcoma is immune reactive to S100 protein and D2-40. Chondrosarcoma is immune non-reactive to cytokeratin [3,4]. Chondrosarcoma requires segregation from neoplasms such as chondroblastic osteosarcoma, enchondroma, fracture callus or chondromyxoid fibroma [3,4]. Chondrosarcoma can be appropriately discerned with pertinent imaging techniques as plain radiographs, computerized tomography (CT) and magnetic resonance imaging (MRI) along with cogent surgical tissue sampling (Tables 1 & 2).
Radiographic assessment of low-grade neoplasms is mandatory and beneficial [3,4]. Upon plain radiography, tumour exhibits popcorn-like calcification manifesting as punctate or ring-like opacities, lytic lesions, endosteal scalloping, thickened bony cortex with cortical erosion or destruction and incrimination of circumscribing soft tissue [3,4].
Cortical destruction and infiltration of surrounding soft tissue within pre-existing enchondroma may indicate emergence of secondary central chondrosarcoma [3,4]. Secondary peripheral chondrosarcoma exhibits a dense cartilaginous cap exceeding >2-centimetre thickness. Periosteal chondrosarcoma denominates a multi-lobulated countenance [3,4]. Computerized tomography and magnetic resonance imaging are beneficial in assessing extent of tumour [3,4].
Chondrosarcoma is appropriately treated with surgical extermination of neoplasm along with removal of broad perimeter of uninvolved tissue. Low grade chondrosarcoma may be completely alleviated with precise surgical intervention [3,4]. Generally, chondrosarcoma is resistant to chemotherapy and radiotherapy although cartilaginous neoplasms mandate intense radiation therapy [3,4]. Surgical extermination of highgrade chondrosarcoma followed by adjuvant radiotherapy is accompanied by superior outcomes. Prognostic outcomes are contingent to factors such as histologic grade, extra-compartmental tumour dissemination and localized tumour reoccurrence [3,4]. Atypical cartilaginous tumour or chondrosarcoma grade I is locally aggressive and exhibits superior prognostic outcome with 5-year proportionate survival at ~85% [3,4]. Chondrosarcoma grade II or grade III enunciate an inferior prognosis with 5-year proportionate survival at ~50%. Localized tumour reoccurrence is contingent to tumour magnitude, adequacy of surgical resection and tumour free tissue perimeter [3,4].
References
- Limaiem F, Davis DD, Kristin L (2022) Chondrosarcoma. Stat Pearls International, Florida, USA.
- Gusho CA, Lee L, Zavras A, Seikel Z, Miller I (2022) Dedifferentiated Chondrosarcoma A Case Series and Review of the Literature. Orthop Rev (Pavia) 14(3): 35448.
- Chen JJ, Chou CW (2022) A Rare Case Report of Mesenchymal Chondrosarcoma with Pancreatic Metastasis. Medicina (Kaunas) 58(5): 639.
- Xie T, Sun Y, Han X, Zhang J (2022) The clinicopathological characteristics and prognosis of young patients with chondrosarcoma of bone. Front Surg 9: 926008.






























