Effects of Different Temperature Treatments on Breaking Dormancy of Pyrus pyrifolia Cultivar ‘Pearl pear’ Seeds
Jialiang Kan1,2, Yufeng Zhang1,3, Yutong Ye1, Hui Li1, Chunxiao Liu1, Jinxing Wang1, Jin Lin1, Yeqing Ying2, Youhong Chang1, Fuliang Cao2,3* and Xiaogang Li1*
1Institute of Pomology, Jiangsu Key Laboratory for Horticultural Crop Genetic Improvement, Jiangsu Academy of Agricultural Sciences, Nanjing, Jiangsu, China
2State Key Laboratory of Subtropical Silviculture, Zhejiang A&F University, Lin”an, Zhejiang, China
3Co-Innovation Center for Sustainable Forestry in Southern China, Nanjing Forestry University, Nanjing, Jiangsu, China
Submission:August 07, 2024;Published: September 06, 2024
*Corresponding author: State Key Laboratory of Subtropical Silviculture, Zhejiang A&F University, Lin”an, Zhejiang, China & Co-Innovation Center for Sustainable Forestry in Southern China, Nanjing Forestry University, Nanjing, Jiangsu, China
Xiaogang Li, Co-Innovation Center for Sustainable Forestry in Southern China, Nanjing Forestry University, Nanjing, Jiangsu, China
How to cite this article: Jialiang K, Yufeng Z, Yutong Y, Hui L, Chunxiao L, at el. Effects of Different Temperature Treatments on Breaking Dormancy of Pyrus pyrifolia Cultivar ‘Pearl pear’ Seeds. JOJ Hortic Arboric. 2024; 4(5): 555648. DOI: 10.19080/JOJHA.2024.04.555648.
Abstract
Temperature is a key factor that influences the breaking of pear seed dormancy. The seeds of Pyrus pyrifolia (sand pear) cultivar, ‘Pearl Pear’ were used to investigate the effects of temperature on seed dormancy breaking. The seeds were cultured at stable temperatures of 4, 8, and 12℃ and variable temperatures of 8-4-8 and 4-8-12℃. According to the results, significant differences were observed in seed germination and growth parameters (germination, rooting, and cotyledon expansion rates) after exposure to a variable temperature treatment of 4-8-12℃. Cotyledon expansion rate of ‘Pearl pear’ seeds treated at 4-8-12℃ was the highest and seedlings grew well after germination. The 4-8-12℃ variable temperature treatment can be used as an optimum temperature treatment for breaking the dormancy of ‘Pearl pear’ seeds. This study could provide a practical and theoretical framework for breeding pears and other fruit trees, forest trees, as well as the production of seedlings.
Keywords:Pyrus pyrifolia; Seed dormancy; Tempetature; Germination; Grow
Introduction
Pear (Pyrus spp.) is one of the most commercially important fruit trees and is widely cultivated in temperate regions of the world [1]. The area under pear tree cultivation continues to expand due to a high planting efficiency, which facilitates agricultural planting structure adjustment [2]. Pears are rich genetic resources with more than 2000 pear varieties. The main cultivated pear varieties are divided into five categories: White, Sand, Qiuzi, Xinjiang, and Western pears [3]. Sand pear is mainly cultivated in the middle and lower reaches of Huai River, Yangtze River, and South China Sea. As a crucial germplasm resource, sand pear is not only economically beneficial, but also an important parental material for breeding new pear varieties [4].
Pyrus pyrifolia cultivar ‘Pearl pear’, which is an early maturing sand pear variety bred by the Shanghai Academy of Agricultural Sciences in China, is an interspecific hybrid variety. The female parent is sand pear variety ‘Yakumo’ from Japan, whereas the male parent is an early maturing pear variety ‘Beurre Giffard’ from France [5]. ‘Pearl pear’ matures early and the fruit develops in 73 days. The maturity period of the pear in Nanjing, China, is from mid to late June. ‘Pearl pear’ has a small fruit, with a single fruit weighing up to 90 g and containing only 10.2% of the soluble solids. Heterosis typically occurs in pear and parents with rich genetic diversity are more likely to obtain ideal offspring [6]; Therefore, ‘Pearl pear’ can be used as an intermediate genetic material to improve pear varieties. However, embryo development in hybrid offspring of ‘Pearl pear’ is incomplete, the embryo contains few substances, and seedling emergence rate is low. Therefore, obtaining sufficient seedlings using conventional methods is a challenge, which limits the utilization of ‘Pearl pear’ [7].
The dormancy of pear seeds enables them to cope with adverse environmental conditions, and to promote the survival and reproduction of pear populations; however, the phenomenon remains unclear [8]. The reasons for pear seed dormancy are complex, and primarily include incomplete embryo development, embryo dormancy, and mechanical restriction of seed coats, which lead to low seed germination rates and uneven seedling emergence [9-11]. Present studies on breaking dormancy and promotion of pear seed germination have focused on low temperature stratification, hormone, and seed coat breaking treatments, among other methods [2,11]. Few studies have investigated the effects of different temperature treatments on breaking the dormancy of pear seeds. Temperature is essential for breaking seed dormancy, as well as promoting seed germination and growth. Generally, high ambient temperatures can increase seed dormancy, whereas low temperatures are conducive to promoting the release of seed dormancy. Some plants can germinate at a specific temperature, while some plants require varying ambient temperatures to facilitate dormancy release [12,13]. Different temperature treatments have varied effects on breaking seed dormancy. Wang applied various low-temperature stratification treatments to Ilex pubescens seeds and observed a seedling emergence rate of 28.8% after two months of cold stratification at 0℃, whereas seed germination rate was 92.0% after cold stratification at 4℃ [14]. Xianliang et al. observed relatively high seed germination rates after the seeds of 12 shrubs were treated at variable temperatures of 5-25℃ [14].
In this study, the seeds of sand pear variety ‘Pearl pear’ were used to investigate the effects of different temperature treatments on breaking seed dormancy and on seedling growth after germination. First, the weights and water absorption characteristics of ‘Pearl pear’ seeds were evaluated. Different stable and variable temperature gradients were applied to break the dormancy of ‘Pearl pear’ seeds. The results of this study could provide a practical and theoretical framework for fruit and forest tree breeding, and seedling production.
Materials and Methods
Plant Material Collection and Disinfection
The new variety of high quality pear, ‘Pearl pear’ was used as the experimental material. The fruits were collected in July 2022 from the fruit tree germplasm nursery of the platform for the protection and utilization of agricultural germplasm resources in Jiangsu Province, Xuanwu District, Nanjing City, Jiangsu Province. The fruits were stored at 25℃ for a week. After completion of the fruit ripening process, the fruits were cut into smaller pieces and the seeds were removed. The seeds were washed with water and dried under a shade at room temperature for subsequent use.
Determination of the Thousand Grain Weight
A total of 100 naturally dried and plump ‘Pearl pear’ seeds were randomly selected, and the thousand grain weight was measured using a precision electronic balance. The procedure was repeated three times.
Determination of Seed Imbibition
A total of 30 naturally air dried, whole, and plump seeds were randomly selected and soaked in water at 25℃ after weighing. Thereafter, the seeds were removed from water after 2, 4, 6, 8, 10, 12, 14, and 16 h of soaking and their weights measured after removing moisture from their surfaces using filter papers. The procedure was repeated three times and seed imbibition was calculated as follows: seed water absorption = (weight of seeds after water absorption - dry seed weight)/dry seed weight × 100%.
Breaking Seed Dormancy using Different Temperature Treatments
Stable temperature (4, 8, and 12℃) and variable temperature (8-4-8 and 4-8-12℃) treatments were used as the test conditions for breaking ‘Pearl pear’ seed dormancy. The total duration of each temperature treatment was 45 days and temperature treatment at 25℃ was used as the control. The ‘Pearl pear’ seeds were washed with clean water, disinfected with 75% ethanol for 1 min, washed 3-5 times with sterile water, and then soaked in sterile water for 14 h. After the seeds absorbed water, wet filter papers were used to evenly distribute the seeds in the Petri dishes. Each Petri dish contained 100 seeds and each temperature treatment was repeated three times. To avoid contamination, the seeds were cleaned and the filter papers were replaced every five days.
Evaluation of Growth Characteristics of Seedlings
The seeds stored in wet Petri dishes were removed and placed
in a culture room at 25 ℃ to promote germination. The number
of germinated seeds was counted after 2, 4, 6, 8, and 10 days. In
addition, rooting rate, cotyledon expansion rate, root length, length
of the aboveground parts, and weight of individual seedlings after
germination were determined on the 10th day. The formulas used
to calculate each parameter were as follows:
germination rate = (number of germinated seeds/number of
tested seeds) × 100%
Germination potential = (number of germinated seeds on the
4th day/number of tested seeds) × 100%
Germination index = Σ(Gn/Dn)
where Gn is the germination potential of a seed on the nth day and Dn is the number of days counted after planting.
Average germination time = Σ(Dn)/Σn
where D is the number of days counted from the beginning of germination and n is the number of germinated seeds on the corresponding days.
Rooting rate = number of seedlings with root lengths > 0.5 cm/total number of tested seeds × 100%
Cotyledon expansion rate = number of seedlings with expanded cotyledons/total number of tested seeds × 100%.
Statistical Analysis
Data were analyzed using MS Excel (Microsoft Corp., Redmond, WA, USA) and IBM SPSS Statistics (IBM Corp., Armonk, NY, USA).
Results
Thousand Grain Weight and Seed Imbibition Characteristics
The thousand grain weight of ‘Pearl pear’ seeds was approximately 19.51 g after natural shade drying and the seeds were relatively complete and rich in nutrients. According to the results of water absorption characteristics of ‘Pearl pear’ seeds (Figure 1), water absorption efficiency was the highest between 4 and 6 h and water absorption rate was 21.09%. The water absorption rate of ‘Pearl pear’ seeds decreased between 12 and 16 h. The degree of protoplast hydration tended to be saturated and seed weights became stable, approximately 4.21 g, which was 2.15-fold higher than that observed before water absorption.
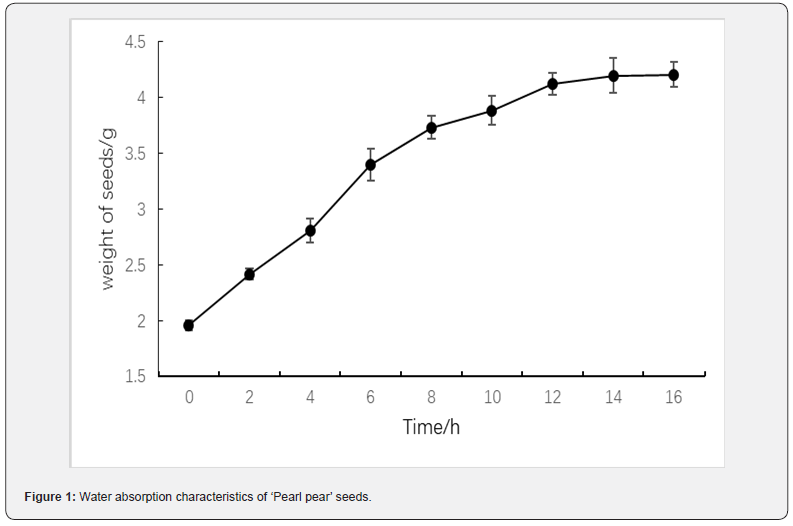
Effects of different temperature treatments on seed germination
The results of the effects of different temperature treatments on the germination of ‘Pearl pear’ seeds are shown in Table 1. The germination rates of treated seeds initially increased gradually with an increase in time and then remained stable. The germination rates of seeds in each treatment was the highest on the 10th day; seed germination rates in the variable temperature treatment of 4-8-12℃ were significantly higher (76.43%,P<0.05) than those in other temperature treatments, followed by the germination rates of seeds in the stable temperature treatment of 8℃ (56.10%) and variable temperature treatment of 8-4-8℃ (41.76%). The germination rates of seeds treated at stable temperatures of 12℃ and 4℃ were 26.42% and 19.34%, respectively. The germination rate of seeds treated at a constant temperature of 25℃ (control) was 0. The germination rates of ‘Pearl pear’ seeds in each treatment are shown in Figure 2.
In addition to germination rates, significant differences were observed in the germination potential, germination index, and average germination time of ‘Pearl pear’ seeds (P<0.05) after exposure to the different temperature treatments (Table 2). The germination potential (11.50%) and germination index (9.53%) of ‘Pearl pear’ seeds in the variable temperature treatment of 4-8-12℃ were the highest. The germination rate and potential of seeds treated at constant temperatures were lower than those of seeds treated at variable temperatures. In addition, the average germination time varied among the treatments. The average germination time of seeds treated at 4-8-12℃ was relatively short (3.75 days), whereas that of seeds treated at 4℃ was the longest (6.20 days). The average germination time of seeds treated at 8℃ was the shortest (3.69 days). However, the germination rate and index of seeds treated at 8℃ were significantly lower than those of seeds treated at 4-8-12℃.
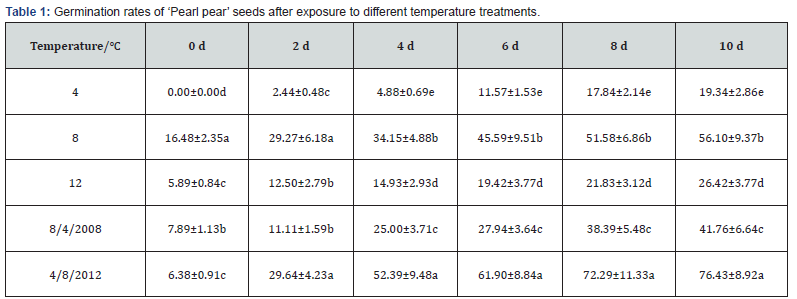
Note: Different lowercase letters within the same column indicate significant differences (P<0.05) among treatments.
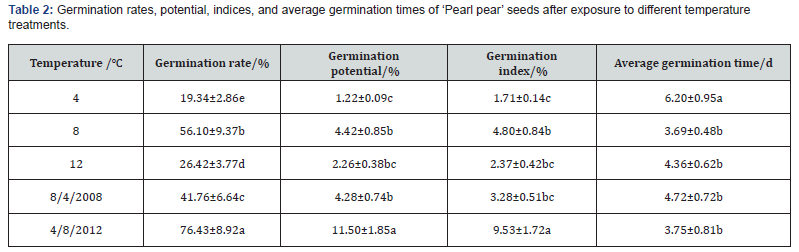
Note: Different lowercase letters within the same column indicate significant differences (P<0.05) among treatments.
Effects of Different Temperature Treatments on Seedling Growth and Development after Germination
The growth characteristics of seedlings on the 16th day after germination are presented in Table 3. According to the results, rooting and cotyledon expansion rates of ‘Pearl pear’ seeds treated at 4-8-12℃ were the highest, with values of 76.67% and 45.83%, respectively, being observed, followed by those of seeds treated at 8℃ (rooting and cotyledon expansion rates of 61.67% and 35.83%, respectively). The rooting rates of seeds treated at 12℃ and 4℃ were 28.94% and 22.37%, respectively, and cotyledon expansion rates at 12℃ and 4℃ were 26.29% and 20.65%, respectively.
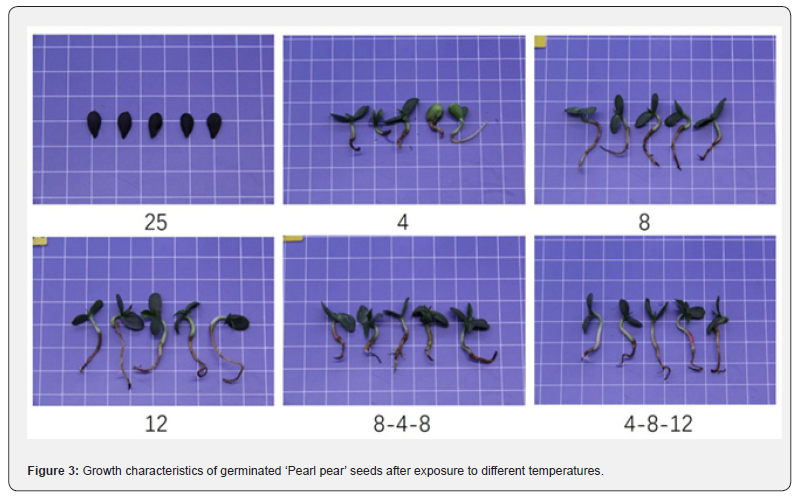
Significant differences were observed in the growth of roots and aboveground parts of seedlings, and the weight of individual seedlings (Figure 3). The growth of seeds germinated at 12℃ was significantly higher than that of seeds in other temperature treatments. The average root length was 4.27 cm, aboveground part length was 4.06 cm, and the average weight of an individual seedling was 193.06 mg; however, the seedlings were thin and weak. The growth of seeds treated at a variable temperature of 4-8-12℃ was relatively low, the average root length was 3.03 cm, aboveground part length was 2.11 cm, the average weight of an individual seedling was 154.68 mg, and the seedlings were strong. The growth of seeds treated at a constant temperature of 4℃ was the lowest, the average root length was 1.38 cm, aboveground part length was 0.71 cm, the weight of an individual seedling was 99.01 mg, and the seedlings were weak.
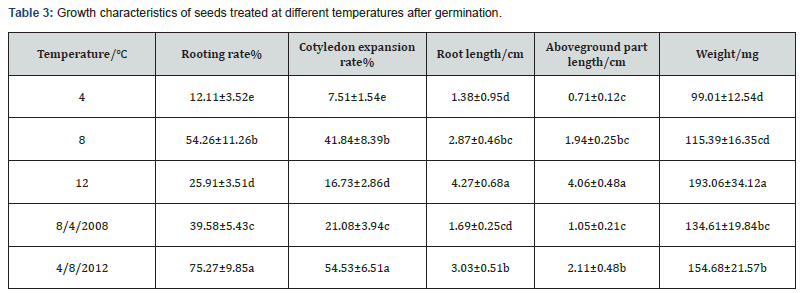
Note:Different lowercase letters within the same column indicate significant differences (P<0.05) among treatments.
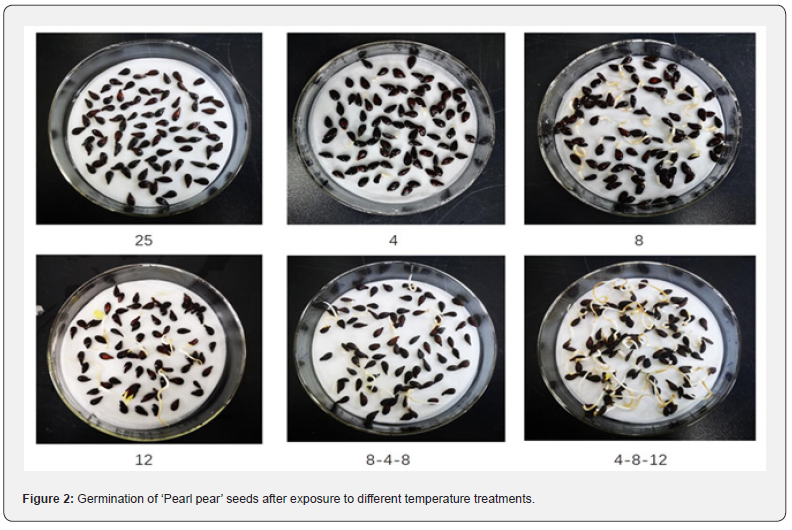
Discussion and Conclusion
Seed dormancy, which enables plants to avoid adverse climatic conditions and maintain plant populations through reproduction, is a common phenomenon in nature [15]. The release of seed dormancy is a complex process. Seeds can adjust their own material transformations by sensing subtle external environmental changes and then regulate the dormancy state to prepare them for germination under appropriate conditions [16,17]. Cell membrane permeability and metabolic enzyme activities in seed embryos increase after the seeds imbibe water, which is conducive to breaking dormancy and promoting germination. In this study, the weights of ‘Pearl pear’ seeds gradually became stable after 12 h of water absorption. The water absorption rate of seeds reduced due to the seed coats that were less permeable to water, and seed weights remained unchanged thereafter [18]. Therefore, seeds should be soaked for 14 h in subsequent temperature and hormone tests to ensure maximum absorption of water by seeds, which is conducive to dormancy breaking and promotion of seedling germination at later stages.
Seed germination is an important process in plant ontogeny and the initial stage of the plant life cycle. Temperature, light, water, among other factors in the external environment, can affect seed dormancy and regulate seed germination [12,19]. Li et al. found that the germination rate of Pyrus betulifolia seeds was 80% after cold stratification at 4℃ for 30 days [20]. Wang et al. observed that subjecting early maturing sand pear cultivars, ‘Cuiguan’ and ‘Cuiyu’ seeds to a combination of low temperature and shelling treatment enhanced breaking of seed dormancy and improved the germination rate of the seeds [11]. In this study, we observed that the germination rate of seeds treated at 25℃ was 0%, suggesting that low temperature was a key factor influencing the breaking of ‘Pearl pear’ seed dormancy, which is consistent with the findings of a previous study on pears [21]. The germination rate of ‘Pearl pear’ seeds after exposure to different temperature treatments initially increased with an increase in germination time, then became stable, and reached a maximum rate on the 10th day.
The germination rates, potential, and indices of seeds treated at a variable temperature of 4-8-12℃ were the highest, whereas the average germination time was short. The effect of the 4-8-12℃ treatment on seed germination was greater than those of stable temperature treatments of 4, 8, and 12℃. The results suggested that a variable temperature treatment was more suitable for breaking seed dormancy and promoting seed germination than single temperature treatments. The variable temperature treatment had a substantial effect on dormancy release of late ripening and hard seeds, which is consistent with the findings of previous studies on temperature treatment effects on breaking the dormancy of Carex spp, Schisandra chinensis, and Heracleum moellendorffii [22-24]. The 4-8-12℃ treatment had a positive effect on seed germination and seedling growth after germination was robust. Seedlings emerged evenly, rooting and cotyledon expansion rates were high, and the survival rate of young seedlings after refinement and transplantation was 96% after ‘Pearl pear’ seeds were exposed to the 4-8-12℃ treatment. The results suggested that a variable temperature treatment was more suitable for promoting metabolism and conversion of storage substances in ‘Pearl pear’ seeds. The 4-8-12℃ treatment simulated the natural temperature changes occurring during seed germination, which enhanced enzyme activity in seed embryos, promoted various physiological and biochemical processes, facilitated the conversion of substances stored in seed embryos, and promoted embryo germination and growth [25-27]. In addition, variable temperature treatments accelerated the mechanical changes in seed embryos and increased seed membrane permeability, which promoted seed germination [28,29].
The 4℃ stable temperature treatment had an insignificant effect on breaking the dormancy of ‘Pearl pear’ seeds. The germination rate of seeds treated at 4℃ was low, the average germination time was long, and seedling germination was uneven. The 8℃ stable temperature treatment had a moderate effect on the germination of ‘Pearl pear’ seeds, which was more suitable for breaking the dormancy of ‘Pearl pear’ seeds and promoting germination than that of the 4℃ treatment. The observation may be because the climate of sand pear producing areas in southern China is mild and humid, and the temperature for breaking the dormancy of sand pear seeds under natural conditions is slightly higher than that in northern China. Therefore, the treatment time of 8℃ can be appropriately extended in subsequent variable temperature treatments. The effect of the 8-4-8℃ variable temperature treatment on seed germination and growth was lower than that of the 8℃ stable temperature treatment, which could be because the low temperature treatment of 4℃ prevented the conversion of storage substances in seed embryos and was not conducive to seed germination. The average germination time of ‘Pearl pear’ seeds treated at 12℃ was the shortest and seedling growth was the highest; however, the germination rate of the seeds was low, seedling roots and stems were slender, and seedlings were weak. Furthermore, the survival rate of seedlings after seedling refinement and transplantation was lower than that of seedlings in other treatment groups. The effect of the 12℃ treatment on breaking seed dormancy was insignificant, although the treatment promoted growth at later stages. Therefore, the duration of the 12℃ treatment could be appropriately decreased during subsequent variable temperature treatments. The effects of the temperature combinations used in this study on promoting the breaking of ‘Pearl pear’ seed dormancy were in the order of 4-8-12℃ > 8 ℃ > 8-4-8℃ > 12℃ > 4℃ > 25℃.
Dormancy release and seed germination are closely associated with external environmental conditions and are the result of natural evolution over time [30]. Low temperature is a key factor that influences seed dormancy, affects changes associated with enzyme activities in seed embryos, affects water absorption by seeds, respiration, nutrient transformations and other metabolic activities, and ultimately affects seed germination and growth [31,32]. This study investigated the effects of different temperature treatments on breaking the dormancy of ‘Pearl pear’ seeds and subsequent seedling growth. The results revealed that a variable temperature treatment of 4-8-12℃ was effective for breaking the dormancy of ‘Pearl pear’ seeds, thereby providing a reliable and practical method of breeding new pear varieties. However, further studies should be conducted to determine nutrient and hormonal changes in seed embryos during the breaking of seed dormancy by fluctuating temperatures, and to elucidate the physiological processes and molecular mechanisms associated with seed dormancy breaking [33,34].
References
- Liu C, Li HL, Ren AH, Chen GY, Ye WJ, et al. (2023) A comparison of the mineral element content of 70 different varieties of pear fruit (Pyrus ussuriensis) in China. Peer J 11: e15328.
- Zhang J, Qian JY, Bian YH, Liu X, Wang CL (2022) Transcriptome and Metabolite Conjoint Analysis Reveals the Seed Dormancy Release Process in Callery Pear. Int J Mol Sci 23(4).
- Zhang JY, Li JM, Xue C, Wang RZ, Zhang MY, et al. (2021) The Variation of Stone Cell Content in 236 Germplasms of Sand Pear (Pyrus pyrifolia) and Identification of Related Candidate Genes. Hortic Plant J 7(2): 108-116.
- Shi CH, Wang XQ, Xu JF, Zhang YX, Qi BX, et al. (2021) Dissecting the molecular mechanism of russeting in sand pear (Pyrus pyrifolia Nakai) by metabolomics, transcriptomics, and proteomics. Plant J 108(6): 1644-1661.
- Kan JL, Liu CX, Wang JX, Wang ZH, Chang YH, et al. (2020) Introduction and cultivation techniques of Pearl pear, a very early maturing pear variety, in Nanjing. South China Fruits 49(5): 123-125.
- Liu SM, Ye G, Richards SM, Smith KF (2009) Segregation and transmission of host resistance to scab (Venturia pirina) in pear breeding progeny under natural infection in an orchard. Scientia Hortic 120(2): 222-229.
- Cantabella D, Dolcet-Sanjuan R, Casanovas M, Solsona C, Torres R, et al. (2020) Inoculation of in vitro cultures with rhizosphere microorganisms improve plant development and acclimatization during immature embryo rescue in nectarine and pear breeding programs. Scientia Hortic 273(1): 1-13.
- Cerovic R, Aksic MF, Dordevic M, Meland M (2020) Functionality of Embryo Sacs in Pear Cultivars 'Ingeborg' and 'Celina' as Related to Fruit Set under Nordic Climate. Plants-Basel 9(12): 1-12.
- Bao JP, Zhang SL (2010) Effects of seed coat, chemicals and hormones on breaking dormancy in pear rootstock seeds (Pyrus betulaefolia Bge. and Pyrus calleryana Dcne.). Seed Sci Technol 38(2): 348-357.
- Liu X, Huang X, Kong XX, Zhang J, Wang JZ, et al. (2020) Sucrose synthase is involved in the carbohydrate metabolism-based regulation of seed dormancy release in Pyrus calleryana Decne. J Hortic Sci & Biotech 95(5): 590-599.
- Wang YZ, Dai MS, Zhang SJ, Shi ZB (2013) Exploring the hormonal and molecular regulation of sand pear (Pyrus pyrifolia) seed dormancy. Seed Sci Res 23(1): 15-25.
- Liyanage GS, Ooi MKJ (2017) Do dormancy-breaking temperature thresholds change as seeds age in the soil seed bank? Seed Sci Res 27(1): 1-11.
- Ooi MKJ, Auld TD, Denham AJ (2012) Projected soil temperature increase and seed dormancy response along an altitudinal gradient: implications for seed bank persistence under climate change. Plant and Soil 353(1-2): 289-303.
- Cui XL, Bi YC, Jiang HZ, Luo YL (2014) Effect of storage and temperature on seed germination of 12 shrub species from the eastern Qinghai-Tibet Plateau. Chinese J Ecol 33(1): 23-32.
- Baskin JM, Baskin CC (2004) A classification system for seed dormancy. Seed Sci Res 14(1): 1-16.
- Meisert A (2002) Physical dormancy in Geraniaceae seeds. Seed Sci Res 12(2): 121-128.
- Tiwari AK, Tiwari TN, Prasad SR (2016) Seed dormancy in ornamental plants: A review. Indian J Agri Sci 86(5): 580-592.
- Kildisheva OA, Dixon KW, Silveira FAO, Chapman T, Di Sacco A, et al. (2020) Dormancy and germination: making every seed count in restoration. Restor Ecol 28: 256-265.
- He XQ, Wang YR, Hu XW, Baskin CC, Baskin JM, et al. (2016) Seed dormancy and dormancy-breaking methods in Leymus chinensis (Trin.) Tzvel. (Poaceae). Grass and Forage Sci 71(4): 641-648.
- Li L, Wen L, Wang G, Lyu Y, Yang Z, et al. (2022) Seed Transmission of Three Viruses in Two Pear Rootstock Species Pyrus betulifolia and P. calleryana. Viruses 14(3): 1-14.
- Kan JL, Zhang YF, Wang JX, Wang ZH, Yang QS, et al. (2022) Effect of different temperature treatment on breaking dormancy of early ripening pear Sucui 1 seed. Jiangsu Agric Sci 50(13): 147-152.
- Granzow-de la Cerda I, Arellano G, Brugues M, Sola-Lopez A (2016) The role of distance and habitat specificity in bryophyte and perennial seed plant metacommunities in arid scrubland fragments. J Vegetation Sci 27(2): 414-426.
- Liu SY, Jiang XM, Liu ZM, Cheng Y, Sun TY, et al. (2019) Mechanism of the Breaking of Seed Dormancy by Flower Thinning in Heracleum moellendorffii Hance. J Plant Growth Regul 38(3): 870-882.
- Yin L, Zhong FL, Zhao CY, Liu SJ, Xiao H (2017) The modulatory effect of Schisandra chinensis seed extract on cisplatin induced cytotoxicity and cell proliferation inhibition in human osteosarcoma cells. Biomed Res India 28(4): 1851-1854.
- Jiao YT, Li ZQ, Xu KY, Guo YR, Zhang C, et al. (2018) Study on improving plantlet development and embryo germination rates in in vitro embryo rescue of seedless grapevine. New Zealand J Crop and Horticul Sci 46(1): 1-12.
- Miransari M, Smith DL (2014) Plant hormones and seed germination. Environmental and Experimental Botany 99: 110-121.
- Stojicic D, Janosevic D, Uzelac B, Budimir S (2008) Factors Influencing Germinnation And Gowth of Isolated Embryos of Pinus Heldreichii. Arch Biol Sci 60(4): 673-679.
- Afzal O, Hassan FU, Ahmed M, Shabbir G, Ahmed S (2022) Temperature Affects Germination Indeces of Safflower (Carthamus Tinctorius L.). J Animal and Plant Sci-Japs 32(6): 1691-1702.
- Cochrane A (2019) Effects of temperature on germination in eight Western Australian herbaceous species. Folia Geobotanica 54(1-2): 29-42.
- Finch-Savage WE, Leubner-Metzger G (2006) Seed dormancy and the control of germination. New Phytologist 171(3): 501-523.
- Eberle CA, Forcella F, Gesch R, Peterson D, Eklund J (2014) Seed germination of calendula in response to temperature. Industrial Crops and Products 52: 199-204.
- Song Y, Li XH, Zhang MY, Xia GW, Xiong C (2023) Effect of cold stratification on the temperature range for germination of Pinus koraiensis. J Forestry Res 34(1): 221-231.
- Liying W (2008) Study on propagation technology and biological characteristics of several species of native Ilex of Zhejiang province. Zhejiang Forest Univ 41-52.
- Shaoling Z, Ming Q, Hao Y, Xiugen LI, Jun WU, et al. (2018) Pedigree Analysis of Pear Varieties(Lines)Bred in China. Acta Horticulturae Sinica 45(12): 2291-2307.






























