Trees with a Denser Crown have Lower Water Consumption than Trees with a Sparser Crown
Annika Wuolo1 and Ann-Mari Fransson2*
1Swedish University of Agricultural Sciences, Landscape Architecture, Planning and Management Management, Design and Construction, Sweden
2The Linneaus University, Forest, and Wood Science, Sweden
Submission: December 8, 2023;Published: December 18, 2023
*Corresponding author: Ann-Mari Fransson, The Linneaus University, Forest and Wood Science, Sweden, Email: ann-mari.fransson@lnu.se
How to cite this article: Annika W, Ann-Mari F. Trees with a Denser Crown have Lower Water Consumption than Trees with a Sparser Crown. JOJ Hortic Arboric. 2023; 4(2): 555632. DOI: 10.19080/JOJHA.2023.04.555632.
Abstract
Trees have many positive effects on the urban environment, but they are also exposed to several stress factors. Water deficit is one of these. Irrigation, structural soils or selecting drought tolerant species are different ways to meet this problem. Another way might be choosing individuals that could reduce water loss due to microclimate differences within the crown. The objective of this study is to address whether different crown densities in single trees may influence the water consumption during days with high atmospheric demand. Two pairs of similar Tilia cordata Mill. ‘Green spire’ with different crown densities, one dense and one sparse, were planted in containers and placed on scales at a paved yard in Alnarp, Sweden, during July and August 2009. Daily weight loss was continuously logged, and measurements of stomatal conductance and stem water potential were performed twice a day. Stomatal conductance was approximately 66% higher (3 mm s-1) in the sparse tree of pair S7:D46 and approximately 20% higher (1 mm s-1) in the sparse tree of pair S52:D21. The dense trees and sparse tree S52 used on average 10.2-10.9 kg water day-1 (1.2-1.4 mm day-1). Sparse tree S7 used on average 13.8 kg water day-1 (2.1 mm day-1). This difference might be caused by different microclimates in the crowns of the sparse and dense trees. Other factors were kept as similar as possible within each pair. The difference in water use was, however, more pronounced in one of the pairs. This could be caused by the different ability for water uptake and transport in the two pairs. The pair with the largest difference in water use had the strongest growth. We conclude that crown density affects water use of a tree in an urban environment; sparse linden trees consume more water than dense Tilia cordata trees.
Keywords: Urban Tree; Tilia Cordata Mill. ‘Green spire’; Microclimate; Stomatal conductance
Abbreviations: T: Temperature; RH: Relative Humidity; PAR: Photosynthetic Active Radiation; SWP: Stem Water Potential; LAI: Leaf Area Index
Introduction
Trees have many positive effects in urban environments, both social and environmental. Besides the aesthetic value of amenity trees, the presence of trees in the urban environment is desirable because of their positive effects on public health Bjork et al. [1] and their ability to capture carbon Nowak & Crane, [2]. Several studies have also shown the vegetation’s ability to mitigate the urban climate Chandler, [3] Eliasson & Upmanis, [4] Svensson, [5] Streiling & Matzarakis [6]. Trees in urban areas reduce the temperature on a local scale both by evapotranspiration and shade Akbari & Konopacki [7]. To be able to fulfil these purposes the trees need to be vital, but many urban trees are exposed to several stress factors that prevent sufficient development, like soil compaction, nutrient- and oxygen deficit, high soil pH, small soil volumes, high air- and soil temperature, pollution and water deficit Craul [8]; Bassuk & Whitlow [9]; Lindsey & Bassuk [10]; Fostad & Pedersen [11].
The importance of water for living organisms is undisputable. From the plants’ perspective water is needed in processes like photosynthesis, as well as for taking up and dissolving nutrients in the soil. The moisture in urban soils is generally lower than in rural soils because of the generally lower water table in urban areas, the high evaporation, the low number of organic molecules, the impermeable ground cover, the high soil compaction Archer & Smith [12], and the intentional and unintentional draining of urban soils caused by the below-ground infrastructure Craul [8]; Lindsey & Bassuk [10]. This low soil moisture is often combined with high atmospheric demand, as temperature generally is higher and air humidity lower in urban compared to rural areas Bassuk & Whitlow [9]. Water deficit is one of the reasons that the life span of urban trees has become shorter Clark & Kjelgren [13]; Lindsey & Bassuk [10]. Different models have been developed to calculate the amount of water that a tree needs, and the soil volume needed to store these amounts of water Kopinga [14], Lindsey & Bassuk [9]; Costello et al.,[15]; DeGaetano [16]. Also, structural soils have been developed to create a space where the requirements of the roots and the need of hard surfaces for traffic are combined Grabosky & Bassuk [17]. These constructions are an expensive compromise, but that is also irrigation. To find other ways to improve the conditions for urban trees there are also reasons to look above ground. Studies of more drought tolerant species used in urban areas are one way of overcoming the low water availability in urban soils and studies of factors affecting water loss in individual trees is another.
Urban vegetation affects the urban microclimate, and vice versa. The performance of green areas for climate regulation depends on the internal characteristics of the vegetated surfaces, such as their evaporative capacity and their organization within the urban setting Shashua-Bar & Hoffman [18]. Evapotranspiration is affected by the microclimate, as temperature, air humidity, light and wind are important for the rate of water loss Monteith & Unsworth [19]; Craul [20]. Water is lost passively through evaporation from surfaces and transpiration from leaves. Some species have leaf characteristics that decrease the evaporative demand close to the leaf surface. Plants can also regulate the rate of water loss through stomatal control, an important species-specific strategy to prevent desiccation. An urban tree planted in a dry site might prevent desiccation by stomatal closure, but when stomata is closed for longer periods, the leaf temperature increases due to lack of evaporative cooling Kjelgren & Montague [21], and the exchange of CO2 and O2 between the air and the leaf is prevented, which leads to a decreased photosynthetic rate Lindsey and Bassuk [1]; Kjelgren & Clark [22]. The organization of trees also affects the urban microclimate. Data from a recent inventory of urban tree databases (Sjöman et al, in press) show that about 123 000 urban trees are used as street trees in the 11 Nordic cities included in the study. Urban trees, especially in paved areas, are mainly planted as single trees and often along a street. The microclimatic conditions within the tree crown of many urban trees therefore vary depending on the shape and size of the individual tree. In a study of Kjelgren & Montague [21], the transpiration in urban trees was divided according to the leaf area of the shaded and sunlit part of the crown, to calculate total transpiration of individual trees. Microclimatic differences cause a difference in transpiration rate between sunlit and shaded parts of the crown, thus the proportion of sunlit versus shaded leaves influence the total transpiration rate in a tree Kjelgren & Montague [21]. During a sunny day, a tree with a sparse crown will have less shaded leaves than a tree with a dense crown. The objective of this study is to address whether different crown densities of individual trees influence their water use during days with high atmospheric demand.
Materials and Methods
Trees
Bare rooted trees (Tilia cordata Mill. ‘Green spire’) with 12-16 cm stem circumference were planted in 500 l wooden containers in spring 2007. The soil used was a sandy loam, 9% clay, 72% sand and 2.4% organic material. Soil porosity was 49 % of the soil volume with an average of 22% air content at field capacity at a ground water level at 50 cm, which corresponded to the situation in the containers. The dry bulk density of the soil was 1.46 g cm-3 and loss of ignition was 4%. The initial soil pH was 8.0 that might be considered normal for the area. An analysis of the initial soil showed an available soil P concentration of 0.175 mg P g-1, 0.065 mg K g-1, and 0.099 mg K g-1 (acetate-lactate extractable soil P, Shuller 1969). The trees were fertilized with 1.5 dl (Blåkorn N-P-K 12-3-13 mikro) each spring from 2008 and 1 dl in June-July from 2007. The containers were placed in a sheltered, paved yard at Alnarp, Sweden (WGS 84: N 55° 39.421', E 13° 5.071') for a minimum of 1 year before the study. The containers were watered during the night when determinations were not made, using a drip irrigation system. Soil moisture was measured at three levels with a profile probe system (PR2, HH2 moisture meter, Delta-T Devices, UK). A tube was inserted on the northeast side of each container, about 0.1 m from the edge. If the soil moisture measurements indicated differences in the amounts of available water between the trees, this was adjusted through irrigation.
Mean yearly precipitation and temperature in the area (Swedish Meteorological Institute) is 50.2 mm and 7.8 C. A local climate station in Alnarp, approximately 350 m from the yard, shows a mean temperature during July-August 2009 of 18.5 C, where midday temperature varied from 15.4 to 26.9 C. Daytime precipitation occurred on 17 days during July to August. Total daytime precipitation was 44mm, and only three days had a precipitation above 2mm. The trees were irrigated to maintain soil moisture of between 17 and 27 volumes % in the root zone. This was estimated to be enough to fulfil their water and oxygen requirements. The containers were painted white to decrease soil temperatures and reduce water uptake from rain and dew. The soil surface was covered with wood chips, and during days of stem water potential measurements the containers were covered with a lid to minimize evaporation from the soil surface. The trees were not pruned after 2006. Data on air temperature (T), relative humidity (RH), solar radiation and VPD was collected from a climate station located approximately 350m from the yard.
Tree growth
Two pairs of trees, one dense and one sparse tree in each pair, were studied to compare the water use of trees with different crown densities. The two trees within a pair were selected as to be as similar as possible in shoot growth, stem growth and leaf size, as these traits are related to the availability of water during the previous and current year. This selection in pairs was to ensure that differences in water use were related to crown density differences. To compare shoot growth between the trees, the length of 4 terminal shoots developed during the current season, without lateral growth at the south side of the crown was determined. Stem circumference was determined at 1 m stem height. In late June the leaf size (Lg) was determined on 3-6 leaves growing on terminal current season shoots at the south side of the crowns (LI-3000 Portable Area Meter, Lambda Instruments Corporation, USA). Total leaf area (LT) was determined towards the end of the season by determining the area of every 10th leaf in the crown (LI-3000 Portable Area Meter, Lambda Instruments Corporation, USA).
Crown density
The crown density was assessed in three different ways: by measuring photosynthetic active radiation (PAR) inside the crowns, by analysing photos, and by measuring crown projection area to calculate leaf area index (LAI). Using a light bar (Sunfleck Ceptometer, Decagon, country) PAR was measured in 2009 at two levels and in four horizontal directions in the crowns under overcast conditions. Photos of the crowns were taken in 2008 using a digital camera from 2-4 different angles. The crown area on the photos were analyzed using ImageJ (http://imagej.nhi.gov/ij version 1.4.3.67, Broken symmetry software, USA) after the photos had been transformed into black and white images by maximizing the intensity of red, green and yellow colors and minimizing the intensity of blue, cyan and magenta colors and increasing the exposure by +1.5 (Adobe photoshop CS4 version 11.0 Adobe systems inc. USA). The crown density was calculated as the area covered by the leaves within a rectangular area where the extension of the longest shoots of the trees were chosen as the outer limits. LAI was calculated by dividing the leaf area with the projected crown area. The projected crown area of 2009 was estimated from measuring the crown radius of each tree in eight directions during 2010 and assuming a circular projected crown area from mean radius of each tree. The radius was then reduced to compensate for the shoot growth of 2010. Because branches do not grow horizontally, and the mean shoot length is calculated from measurements on the south side of the crown, where shoot growth is likely to be highest, the actual crown radius change from 2009 to 2010 is assumed to be one third of the determined shoot growth in 2010.
Water loss
To study total water loss, the four trees in the different pairs were placed on scales (WB-3000, Vetek, Sweden) starting from June 27, 2009, when bud set and apical growth had ceased, to August 31, 2009, when senescence was expected to affect the trees. The weight was logged every 10th second by a computer program (Weigh Soft 1.0, Vetek, Sweden) and the mean weight of each container was then calculated for every 30 minutes period. For calculations of the daily water use (kg) weights derived at 06:00 and 20:00 were used. Days with missing data or with precipitation were excluded, which makes the number of days used for calculations different between the two pairs. To be able to compare the daily and hourly water use of trees with different leaf area, relative water use (mm) was calculated as water loss (kg) per total leaf area (m2). Differences in water loss between the paired trees were analyzed using paired t–test (Minitab® 16 Statistical Software, Minitab Inc., USA).
Stem water potential and stomatal conductance
Stem water potential and transpiration rate affects each other, therefore both SWP (Pump-Up Pressure Chamber, PMS Instruments, USA) and stomatal conductance (Porometer AP4, Delta-T Devices, UK) was determined. Measurements of stem water potential (SWP) are used as a plant water stress indicator McCutchan & Shackel, [19]. For measurements of SWP, 3-4 leaves on the shaded side, situated in the lower part of the crown were bagged with stem water potential bags (PMS Instruments, USA) for at least one hour before measurements. Due to differences in light, temperature and air humidity between different parts of the tree crowns, measurements of stomatal conductance were performed on 5-8 leaves in each of three positions: sunlit, shaded and inner parts of the crowns. Differences in stomatal conductance and SWP between the paired trees were analyzed using paired t–test (Minitab® 16 Statistical Software, Minitab Inc., USA).
Results
Tree growth
The dense tree D46 had the highest growth increase of all trees, with highest shoot- (29.3 cm) and stem growth (2.0 cm), and the largest leaf size (Lg, 60.93 cm2). A sparse tree that had the most similar growth increase was S7 (22.3 cm, 1.5 cm and 40 cm2). The dense tree D21 had less vigorous growth, with a shoot growth of 15.35 cm, a stem circumference increases of 1.4 cm and a leaf size of 42.5 cm2. S52 was a sparse tree with similar growth (10.8 cm, 1.2 cm and 40.8 cm2). The trees were paired as D21:S52 and the stronger growing pair D46:S7.
Crown density
Crown density calculated by PAR measurements, photo analysis and leaf area index (LAI) all showed that the two dense trees were to a various degree denser than the two sparse trees (Table 1). Within pair D46:S7 the difference in crown density ranged from 14 to 53% depending on the method used, and within pair D21:S52 the difference ranged from 10 to 149%. Visually S52 seemed as the tree with the sparsest crown and PAR measurements in the crown as well as photo analysis confirmed this assessment. LAI varied from 4.1 (S7) to 4.97 m2 (D21). The crown projection areas were similar in all trees (1.57-1.82 m2). The trees were approximately the same height.
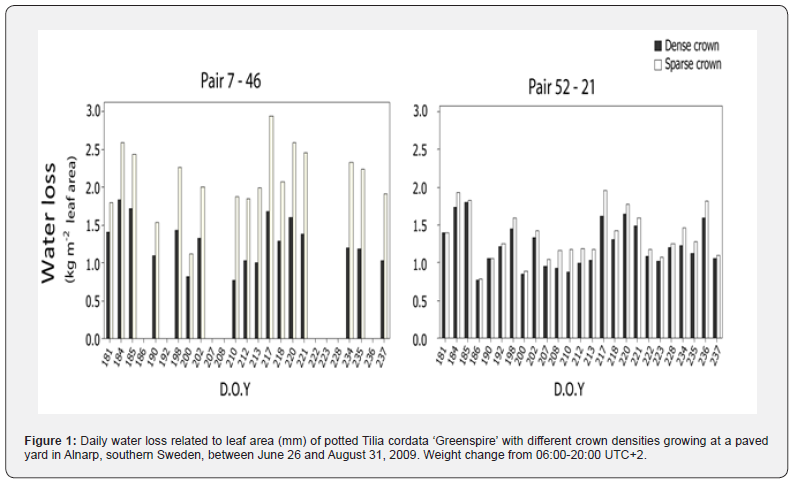
Water loss
Data from 25 days (D21:S52) and 17 days (D46:S7) was used to calculate daily water loss. In both pairs the daily water loss was higher in the sparse than in the dense tree (figure 1 D21:S52, p<0.001, D46:S7, p<0.001, paried t-test). The dense trees and S52 used on average 10.2-10.9 kg/day or 1.2-1.4 mm/day. S7 used on average 13.8 kg/day or 2.1 mm day -1.
Stomatal conductance and stem water potential
The average stomatal conductance in the trees was between 2.1 to 13.9 mm s-1. In both pairs, the stomatal conductance of the sparser tree was higher than in the denser tree both during the AM and PM (Figures 2 & 3, p<0.01, paired t-test). Exceptions were July 4 and August 6, when the average stomatal conductance was higher in D21 than in S52. The stomatal conductance was approximately 66% higher (3 mm s-1) in S7 than in D46 and approximately 20% higher (1 mm s-1) in S52 as compared to D21. Stomatal conductance was also higher at measurements early in the day (AM, 3.76-13.9 mm s-1, figures 2a & 3a) than in the afternoon (PM, 2.1-8.77 mm s-1, figures 2b & 3b p<0.01, paired t-test). Stem water potential (SWP) was between -12.4 and -3.5 bar. There was no difference in SWP between the dense and sparse trees of pair D46:S7, but in pair D21:S52 the sparse tree had a lower SWP in the afternoon (P=0.029, figures 2 & 3).
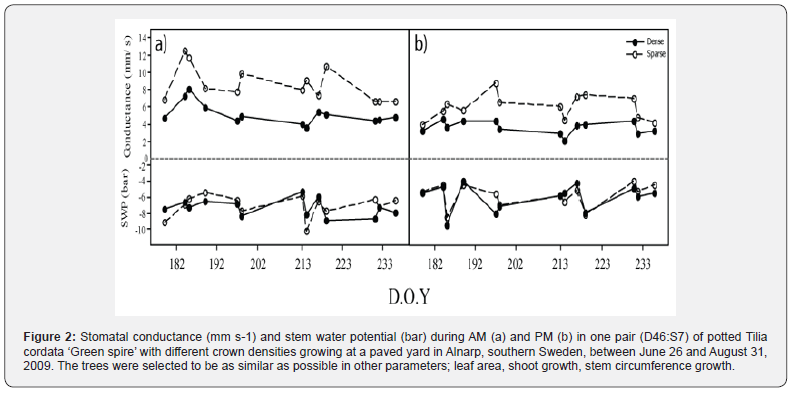
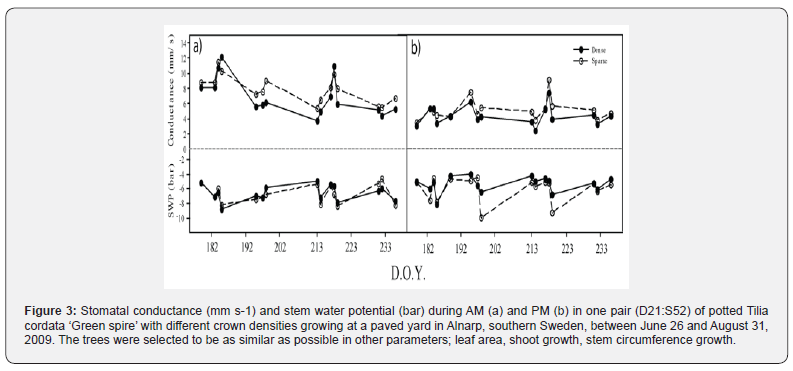
Discussion
We found that the denser trees consumed less water than the sparser trees, which supports our initial hypothesis. Both the water loss and the stomatal conductance were higher in the sparser trees. The higher amount of water leaving the sparser tree seems to be through a higher water loss across the stomata, as the condition at soil surface was similar in all trees, and evaporation from the soil was kept to a minimum through covering the soil surface. If stomata are open, transpiration rate is driven by VPD Monteith & Unsworth [23]; Lindsey & Bassuk [10], but transpiration is also dependent on soil water access and the ability of the tree to take up and transport water from the soil to the atmosphere through stomata Kozlowski & Davies [24]; Lindsey & Bassuk [10]. In this study, differences in available soil water were minimized through continuous irrigation during the night, and differences in water transport ability were minimized through using a clone and combining trees with similar leaf size, shoot- and stem growth in each pair. All four trees were positioned to experience similar ambient conditions, therefore the atmospheric demand driving the transpiration should have been similar, unless there were actual microclimatic differences within the crowns of the different trees. Stomatal conductance and stem water potential (SWP) was measured during warm, calm, sunny days when atmospheric demand was expected to be high. During these conditions the crown density difference of the trees is likely to create the largest differences in the microclimate within the crowns. A larger part of the canopy of a sparse tree should receive direct radiation compared to a dense tree, so there would be more sunlit leaves and less shaded leaves in a sparse than in a dense tree crown. In this study it is therefore likely that the difference in water consumption was caused mainly by differences in the microclimate within the tree crowns.
Stem water potential of the Tilia cordata in our study varied from -3.5 to -12.4 bar, which is like the results of Sellin, Kupper [25] who determined -0.4 to -1.0 MPa (-4 to -10 bar) midday leaf water potential on bagged leaves of larger trees of Tilia cordata. Minimum leaf water potentials of -2.1 MPa (-21 bar) was determined in Tilia cordata during a 24-h period Pigott & Pigott [26]. Their low water potential was due to drought. This indicates that our trees did not experience severe drought. Montague et al. [27] studied water use in newly planted Tilia cordata of a similar size to the trees in our study. Weekly pre-dawn leaf water potential in their trees varied from -0.5 MPa (-5 bar) in the beginning to -0.3 MPa (-3 bar) later in the study. Our trees showed a lower water potential that is due to AM and PM determinations of SWP, when transpiration had been going on for several hours, while Montague et al. [27] measured pre-dawn water potential, when the trees had recovered during night. The variation in SWP resulted in no consistent pattern in relation to time, tree crown density, conductance etc. in the trees, which agrees with the result of Whitlow et al. [28]. In our study stomatal conductance varied from 2.1 to 13.9 mm s-1 (approximately 85 – 560 mmol m-2 s-1). Kjelgren & Montague [21] measured similar stomatal conductance (approximately 1-7.5 mm s-1) in pear (Pyrus callyerana ‘Cleveland Select’) over turf and asphalt. Leaf conductance in understory Tilia cordata trees were 52 - 112 mmol m-2 s-1 Sellin & Kupper [25]. This is approximately in the same range as our results, and the lower stomatal conductance compared to our trees is likely due to the lower light conditions in the understory.
The sparse trees showed higher stomatal conductance than the dense trees. However, on two occasions during AM, July 4 and August 6, the dense tree D21 had higher stomatal conductance than the sparse tree S52. Prior to these dates there had been several warm days with VPD above average, according to data from the nearby climate station. Because of the lack of evaporative surfaces, and heat stored during the preceding warm days, the temperature and VPD was higher still in the paved yard where the trees were situated, than in the more rural environment surrounding the climate station. In addition, soil moisture was slightly lower in pair D21:S52 both on July 4 and August 6, compared to the day before. These conditions might contribute to the effect that the dense tree D21 showed a higher stomatal conductance than the sparse tree S52. The determinations of the daily weight change should show if this higher transpiration in tree D21 last long enough to result in a higher water loss compared to tree S52, but this was not the case. Stomatal conductance was measured only twice a day, AM and PM, and even though the two trees in a pair were measured almost simultaneously, measured conductance is probably not representative for the whole morning or afternoon. It is therefore likely that this peak in stomatal conductance in the denser tree during AM of July 4 and August 6 was temporary. Kozlowski & Davies [24] state that in established trees a lag phase can occur between uptake and transpiration, with the result that plants can experience water stress even when the soil is moist. This agrees with a study where water deficit in street trees was mainly caused by high evaporative demand rather than low water supply Whitlow et al., [28]. If our hypothesis about differences in microclimate between sparse and dense tree crowns is correct, this lag phase should occur earlier in a sparse tree than in a dense tree because of a higher atmospheric demand in the former. Our data does not support this, though, as the level of stomatal conductance of the dense tree D21 during July 6 and August 4 was more like the sparse tree S7 than like the other dense tree. The difference between D21 and S52 does not seem to be caused by a transpiration decrease in S52, but rather by a transpiration increase in D21.
The weight data showed a total daily water use of on average 1.2-2.1 mm/day. This is like the results of Peters et al. [29] showing that deciduous trees transpired on average 1.11 mm/day, and the two Tilia cordata trees included in their study transpired on average 1,87 mm/day. Montague et al. [27] found daily water use in small trees of Tilia cordata of about 4.3 mm/day, varying from 1 to 9 mm/day. This is more than twice the average daily water use of the trees in our study, and climate differences might be the main reason, as their study was performed in a semi-arid climate with an average daily VPD of 2.0 kPa, compared to 0.84 kPa in our study. In another study by Montague et al. [27], where water loss was not measured, but estimated by a standard flux equation, the daily estimated water loss of Tilia cordata ‘Greenspire’ was 0.57-1.86 mm/day during a sunny and a cloudier day respectively. The amounts of water loss are like our result, but in their study the trees used less water during the sunny days than during more cloudy days, which is the opposite in our study. The climate difference is probably the reason for this difference in water use during warm days, as the high VPD measured in their study was likely to induce stomatal closure during warmer days. The VPD in our study was probably not high enough to trigger a similar reaction for longer periods during the day.
If the water loss results from our study is transferred to a tree with a leaf area of 10 m2 this would result in a water consumption of 12-20 liters/day depending on crown density. In an urban environment where rooting space for the trees is scarce, and where the surface is often paved, the amount of water that can be stored in the soil becomes very important for the water status of the tree. A restricted soil volume makes not only the water holding capacity of the soil, but also the rate of water loss important factors for the time span until water deficit occurs. With a water loss of 20 liters/day, a tree would consume 200 liters in 10 days, while a tree that transpires 12 liters/day would consume the same amount in 16 days. Different soils can store very different amounts of water at field capacity due to their structure and texture. For example, clay soil can store approximately 200 liters of plant available water/ m3 soil, while sandy soil can store about half of this amount. To postpone the point in time when drought stress occurs, either the rooted space needs to be large, or watering to replenish the water supply of the soil is needed. This is usually not the case in urban environments. If a tree with a denser crown uses less water than a tree with a sparse crown, the time span between drought stress occasions could be longer for the dense tree, if the total leaf area of the denser tree is not larger. The dense trees and the sparse S52 used on average 10.2-10.9 kg/day or 1.2-1.4 mm/day. The sparse S7 used on average 13.8 kg/day or 2.1 mm/day. S7 had the lowest number of leaves (3500 compared to 4200-4200 in the other trees) and the lowest total leaf area (6.5 m2 compared to 7.5-8.7 m2). In a study by Lindsey & Bassuk [10] 85% of the variability in total water loss could be explained by total leaf area and atmospheric demand. The low leaf area of S7 in our study would not result in higher, but in lower water consumption.
In our study the weight change was logged continuously most days from the end of June to September, and from this data it seems as if the denser trees in general had lower water consumption than the sparser, also on less sunny days. This indicates that the different crown densities might affect water use not only during warm, calm days, but also on days with lower evaporative demand. There is a possibility that there was shade adapted leaves within the denser trees, where the number of stomata per leaf area was lower compared to leaves adapted to sunny conditions, but the trees in the study were probably too small to have developed shade leaves. During summer in Sweden the sun rises early and sets late (e.g. 1 July Malmö 03:30-21:00), no part of the tree is likely in constant shade. It is possible that VPD is different within the tree crowns also during days with a lower atmospheric demand, but it is probably more likely that different light conditions in the tree crowns have affected stomatal aperture, as stomata respond to decreasing light.
The water loss from the trees shows that there is a large difference between the trees in pair D46:S7, and a much smaller difference between D21: S52. Non-paired comparisons between the sparse trees show that tree S52 used less water than tree S7. This makes it difficult to estimate the effect of the tree crown density. Measured growth parameters (shoot, stem and leaf) indicates that tree S7 was able to take up and transpire water at a higher rate than tree S52, and tree S7 sustained this relatively vigorous growth through the years (2008-2010). The root development below ground was likely high, as cell expansion in stem, twigs and leaf is dependent on a good water uptake by the roots. Even if the root system of tree S7 might have been particularly well developed, this does not explain the large difference between sparse tree S7 and dense tree D46, as D46 had an even better growth from 2008 – 2010. In this respect the two trees should have been a good match. From this it seems that the smaller difference between D21:S52 was due to lower water consumption than expected in S52 [31].
Tree S52 had the lowest shoot growth as compared to the other three trees. Mean shoot growth was about 11 cm, which corresponds to about half of the shoot growth of tree S7. Stem circumferences increase and leaf size (Lg) was also lowest in S52 as compared to the other trees. This suggests that the water uptake ability in tree S52 was not as good as in tree S7. The growth parameters in tree D21 were only slightly higher than those of S52, indicating that the water uptake ability of the tree pair D21:S52 was probably not as high as D46:S7. The different methods to determine crown density all agreed with the visual assessment of whether the trees were dense or sparse [32]. The variation in the density methods was however very high. Our study did not address the question of the usefulness of the methods as predictors of water consumption differences, and in practice visual assessment might often be sufficient. Whether this result is valid for Tilia cordata of other sizes or in other sites, or for other species, is probably dependent on the mechanisms behind the difference in water loss rate between dense and sparse trees in the study. If the main reason for water consumption differences was lower light inside the denser tree crown, any tree where stomata is sensitive to decreasing light should transpire less in shaded parts of the crown than in the more exposed parts. From the results of our study, it seems as if light differences in the tree crowns may be an important factor affecting total tree water consumption, as differences in daily water consumption occurred also during less warm days. However, if the main reason for lower transpiration rate was lower VPD within the denser canopy, this could be dependent on several factors. A difference in water loss rate would probably vary with size, shape, species, site and climate. In this study we did not address the question of the impact of VPD on total tree water loss.
Conclusion
The results of this study show that the crown density affects the water use of a tree in an urban environment; sparse little-leaf linden trees consume relatively more water than dense trees. This effect might become more pronounced when the trees are well established, when they are able to take up and conduct water at higher atmospheric demand without stomatal regulation occurring as a response to temporary drought stress.
Acknowledgements
We would like to thank FORMAS and KSLA (Stiftelsen Edvard Nonnens stipendiefond) for funding this project, and Professor Susanne Widell at Lund University for discussions on this paper.
References
- Bjork J, Albin M, Grahn P, Jacobsson H, Ardo J, et al. (2008) Recreational values of the natural environment in relation to neighbourhood satisfaction, physical activity, obesity and wellbeing. J Epidemiol Community Health 62(4): e2.
- Nowak DJ, Crane DE (2002) Carbon storage and sequestration by urban trees in the USA. Environmental Pollution 116(3): 381-389.
- Chandler T J (1976) Urban Climates and Natural-Environment. Int J Biometeorol 20(2): 128-138.
- Eliasson I, Upmanis H (2000) Nocturnal airflow from urban parks-implications for city ventilation. Theoretical and Applied Climatology 66(1-2): 95-107.
- Svensson MK (2002) Urban planning in relation to land use, planning and comfort. Ph.D. Thesis, Earth Science Centre, Gothenburg University, Gothenburg.
- Streiling S, Matzarakis A (2003) Influence of single and small clusters of trees on the bioclimate of a city: A Case study. J Arboriculture 29(6): 309-316.
- Akbari H, Konopacki S (2004) Energy effects of heat-island reduction strategies in Toronto, Canada. Energy 29: 191-210.
- Craul PJ (1985) A Description of urban soils and their desired characteristics. J Arboriculture 11(11): 330-339.
- Bassuk N, Whitlow T (1987) Environmental stress in street trees. Acta Horticulturae 195: 49-57.
- Lindsey P, Bassuk N (1991) Redesigning the urban forest from the ground below: a new approach to specifying adequate soil volumes for street trees. J Arboricultural 15(4): 25-39.
- Fostad O, Pedersen PA (1997) Vitality, variation, and causes of decline of trees in Oslo center (Norway). Journal of Arboriculture 23(4): 155-165.
- Archer JR, Smith (1973) Relation between Bulk Density, Available Water Capacity, and Air Capacity of Soils, J Soil Sci 23(4): 475-480.
- Clark JR, Kjelgren RK (1989) Conceptual and management conciderations for the development of urban tree plantings. J Arboriculture 15(10): 229-236.
- Kopinga J (1991) The effects of restricted volumes of soil on the growth and development of street trees. J Arboriculture 17(3): 57-63.
- Costello LR, Matheny NP, Clark JR (2000) A guide to estimating irrigation water needs of landscape plantings in California. The landscape coefficient method and WUCOLS III, In: University of California Cooperative Extension (C. D. o. W. Resources, ed.), California Department of Water Resources, USA.
- DeGaetano AT (2000) Specification of soil volume and irrigation frequency for urban tree containers using climate data. J Arboriculture 26(3): 142-151.
- Grabosky J, Bassuk N (1996) Testing of structural urban tree soil materials for use under pavement to increase street tree rooting volumes. J Arboriculture 22(6): 255-263.
- Shashua-Bar L, Hoffman ME (2003) Geometry and orientation aspects in passive cooling of canyon streets with trees. Energy and Buildings 35(1): 61-68.
- Monteith JL, Unsworth MH (1990) Principles of environmental physics. (2nd edn).
- Craul PJ (1999) Urban soils: Applications and Practices. Wiley, New York. Soil Sci 165(5): 452-453.
- Kjelgren R, Montague T (1998) Urban tree transpiration over turf and asphalt surfaces. Atmospheric Environment 32(1): 35-41.
- Kjelgren RK, Clark JR (1993) Growth and water relations of Liquidambar-styraciflua L in an urban park and plaza. Trees-Structure and Function 7(4): 195-201.
- McCutchan H, Shackel KA (1992) Stem-Water Potential as a Sensitive Indicator of Water-Stress in Prune Trees (Prunus-Domestica L Cv French). J Am Society Horticultural Sci117(4): 607-611.
- Kozlowski TT, Davies WJ (1975) Control of water balance in transplanted trees. J Arboriculture 1(1): 1-10.
- Sellin A, Kupper P (2007) Effects of enhanced hydraulic supply for foliage on stomatal responses in little-leaf linden (Tilia cordata), Eur J Forest Res 126(2): 241-251.
- Pigott CD, Pigott S (1993) Water as a determinant of the distribution of trees at the boundary of the mediterranean zone. J Ecol 81(3): 557-566.
- Montague T, Kjelgren R, Allen R, Wester D (2004) Water loss estimates for five recently transplanted landscape tree species in a semi-arid climate. J Environ Horticulture 22(4): 189-196.
- Peters EB, McFadden JP, Montgomery RA (2010) Biological and environmental controls on tree transpiration in a suburban landscape. J Geophysical Res Biogeosci 115.
- Montague T, Kjelgren R, Rupp L (2000) Surface energy balance affects gas exchange and growth of two irrigated landscape tree species in an arid climate. J Am Society Horticultural Sci 125(3): 299-309.
- Schuller H (1969) The calcium acetate lactate method. A new method to determine the plant available phosphate in soils. Zeitschrift fuer Pflanzenernaehrung und Bodenkunde 123(1): 48-63.
- Sjöman H, Östberg J, Bühler O (2011) Diversity and distribution of the urban tree population in ten major Nordic cities. Urban Forestry & Urban Greening 11(1): 31-39.






























