Atmospheric Carbon Dioxide, Climatic Change, and the Survival of the Earth’s Biota
Stuart A Harris*
Department of Geography, University of Calgary, Canada
Submission: September 23, 2023; Published: September 28, 2023
*Corresponding author: Stuart A Harris, Department of Geography, University of Calgary, Canada, Email: harriss@ucalgary.ca JOJ Hortic
How to cite this article: Stuart A Harris*. Atmospheric Carbon Dioxide, Climatic Change, and the Survival of the Earth’s Biota. JOJ Hortic Arboric. 2023; 4(1): 555626. DOI: 10.19080/JOJHA.2023.04.555626.
Abstract
Examination of the climatic history of the Earth shows that atmospheric carbon dioxide is not fundamentally a cause of climate change. It is however a fundamental requirement for photosynthesis and every time there is a cold event (glaciation), this gas becomes more soluble in the oceans and becomes depleted in the atmosphere. Its current abundance is precariously low and could result in the failure of plants to be able to carry out photosynthesis during the next cold event. Decarbonization is liable to destroy life on the Earth as we know it. Instead, we need to increase the atmospheric carbon dioxide as far as possible to avoid a catastrophe.
Keywords: Atmospheric carbon dioxide; Glaciation; Photosynthesis; Decarbonization
Introduction
Photosynthesis by plants is the basic supplier of energy to the biota of the Earth. Without it, the plants and animals that depend on the sugars and other more complex carbon compounds for energy to carry out their cycles of life would die off and the planet would look as barren as the other heavenly bodies. This paper will discuss a little-known problem which could spell the end of life on the planet as we know it.
In the Beginning
The Earth formed about 4.0-2.5 Ga B.P (billion years) before the present (B.P.). There are three theories regarding the method of its formation, but all three assume that the planets including the Earth formed by coalescence of a disc of dust and gasses left over from the formation of the Sun swirling around in space in a disc-like plane [1,2]. The sun had already formed in the center of the system and provided solar energy radiating outwards, currently supplying about 99.6% of the energy arriving on the surface of the Earth. In the beginning, the Earth was molten with plumes of gases such as nitrogen, hydrogen, helium, and water vapor being ejected from volcanoes to form the beginnings of the atmosphere. The hydrogen and helium mainly escaped into space at this time as the planet differentiated into layers... The water vapor provided the water that accumulated on the surface as the oceans, while active volcanoes formed the islands of land [3]. Falling rain caused weathering and decomposition of the basalt providing calcium and sodium ions which were washed into the sea [4]. The sodium ions were more electropositive, hence the calcium ions accumulated on the exchange complex of the marine sediments.
Beginning of life on Earth and the Appearance of Carbon Dioxide and Oxygen
There is evidence that life in the sea could have evolved before 4.28 Ga B.P. [5] but it is nonproven. Strong fragmentary evidence obtained indicating that life was present during the Archean Eon (4.0 - 2.5 a B.P.). The strongest evidence is the presence of marine Aragonite deposits together with apparent glacial tills from the Polisanka Sedimentary Formation on the Kola Peninsula dated at 3.8 Ga B.P. Aragonite is a form of calcium carbonate that is deposited in a cold marine environment after oxidation of methane in the atmosphere to form carbon dioxide which then partially dissolves in water where it can combine with Ca++ ions to form the aragonite. To obtain the necessary oxygen, there must have been marine organisms producing it by photosynthesis [6,7]. Photosynthesis is believed to have developed in the Late Archean Era with the appearance of Stromatolites [8]. These micro-organisms build up mats around sand grains. Part of the oxygen would also move into the atmosphere and permit the formation of an ozone layer in the stratosphere which would block out ultraviolet light, so enabling the survival of a wide range of life starting in the Late Pre-Cambrian era that could not otherwise survive.
The oxygen released into the atmosphere could not build up to any significant degree until mineral sinks of unoxidized Sulphur and iron had been exhausted. Until roughly 2.3 Ma B. P., oxygen was probably only 1% to 2% of its current level [9]. Thereafter the oxygen content of the atmosphere increased to about 10%. Banded iron formations composed of hematite, which provide most of the world’s iron ore, are not found in rocks younger than 1.9 Ma, after all the iron in the oceans had been oxidized [9-11].
Thirteen periodic but random cold events started by about 3.9 Ga B.P. After the 6th glaciation at the end of the Precambrian period, there was an explosion of animal life in the oceans. The atmospheric carbon dioxide from about 20% to very low during the succeeding Cambrian period. Carbon dioxide is unusual in becoming more soluble in water at lower temperatures [13]. Conversely, it is degassed when the seas warm up. This explains the parallel reaction of the gas to warming temperatures during the last 50 years. As a chemical substance, carbon dioxide can react with other substances such as calcium ions in the seas to form calcium carbonate and does so in all water bodies where there is an abundant supply of calcium ions in the water. During the late Ordovician and the 60 Ma Karroo Glaciation of the Carboniferous and Permian periods, this reaction with the calcium ions accumulated during the previous 3Ma plus the carbon dioxide used by the terrestrial plants resulted in the depletion of the atmospheric carbon dioxide from to very low levels from which it has never fully recovered (Figure 1). Instead, it reached a maximum of 10% in the middle of the Jurassic period, after which it steadily dropped until the last two glacial events. In both events, it reached within 100 ppm of the lower limit for photosynthesis in plants. It is currently rising because of degassing from the vast volume of water warming in the oceans, augmented by the comparatively miniscule effect of humans. The recent forest fires will also have added to the total though it remains to be seen what effect they really have on the atmospheric carbon dioxide content.
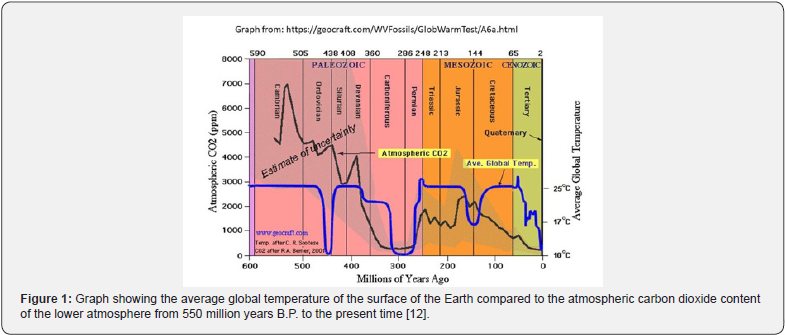
Relation to Government Policies
Unfortunately, Governments are attempting to reduce the total atmospheric carbon dioxide on the assumption that it is the cause of climate change. As Figure 1 shows, this is not the case. If the atmospheric carbon dioxide drops below about 500ppm during one of the next. glaciations, the plants will be unable to carry out photosynthesis and will use up the sugars stored in their tissues to produce seeds before they die. Since they are the ultimate source of food for the rest of the biota, the entire food chain will collapse, and the surface of the Earth will become as barren as that of the other planets [3,14]. For some reason, this is being disregarded by most Governments. The idea of decarbonization needs rethinking unless we wish to risk bringing about the end of humans soon [12,15-17].
References
- Stanley SM (2005) Earth System History. (2nd), Freeman, New York, USA.
- Gradstein FM, Ogg JG, Smith AC (2004) A Geological Time Scale. Reprinted in 2006 with corrections. Cambridge University Press.
- Harris SA (2023) Evolution of the climate of the Earth. Journal of Civil Engineering Research and Technology, 5(2): 1-15.
- Ball EA, Bochnike P, Harrison TM, Wendy LM (2015) Potentially biogenic carbon preserved in a 4.1-billion-year-old zircon. Earth Atmospheric And Planetary Sciences 112(47): 14518-14521.
- Bowman J (2006) The Methanotrophs-The Families Methylococcaceae and Methylocystaceae p. 266-289.
- Kasting JF, Howard M, Tazewell M (2006) Atmospheric composition and climate on the early Earth. Philos Trans R Soc Lond B Biol Sci 361(1474): 1733-1742.
- Brasier A, AP Martin, VA Melezhik, AR Prave (2013) Earth’s earliest glaciation? Carbonate geochemistry and geochronology of the Polisanka Sedimentary Formation, Kola Peninsula, Russia.
- Franch F, (2018) Petrographic and geochemical characterization of the Lower Transvaal Supergroup stromatolitic dolostones (Kange Basin, Botswana). Precambrian Research 310: 93-113.
- Norman WL, (2007) Age of the Earth. USGS Publication Services.
- Halverson GP, Hoffman PR, Schrag DP, Maloop AC (2005) Towards a Neoproterozoic composite carbon isotope record. Geological Society of America Proceedings 117(9-10): 1187-1207.
- Young G M, (2013) Precambrian supercontinents, glaciation, atmospheric oxygenation, metazoan evolution, and impact that may have changed the second half of Earth’s history. Geoscience Frontiers 4(3): 247-261.
- Frakes LA, Francis JE (1988) A guide to Phanerozoic cold polar climates from high-latitude ice-rafting in the Cretaceous. Nature 339: 547-549.
- Humlum O, Stordahl H, Solheim JE (2013) The phase relation between atmospheric carbon dioxide and temperature. Global Climatic Change 100: 51-69.
- Payet P, (2022) The Rational Climate e-book: Cooler is riskier.
- Taylor TN, Taylor E L, Kring M (2008) Paleobotany: The biology and evolution of fossil plants. Academic Press, p. 49.
- Milankovitch M (1920) Théorie Mathématique des Phénomènes Produits par la Radiation Solaire [in French]. Gauthier-Villars, Paris, France.
- Milankovitch M (1941) Canon of Insolation and the Ice-Age Problem [in Serbian]. Royal Serbian Academy, Belgrade, Yugoslavia, Serbia.






























