Effects of Watering Regime and Biochar on the Growth, Biochemical Constituents and Drought Susceptibility of Seedlings of Some Urban Forest Tree Species
Ogunwole Ayodeji A1, Agele Samuel O2* and Ologundudu Folorunsho3
1Department of Biological Sciences, Wesley University, Ondo, Nigeria
2Plant Physiology & Ecology Group, Department of Crop, Soil & Pest Management, Federal University of Technology, Akure, Nigeria
3Department of Biolsogy, Federal University of Technology, Akure, Nigeria
Submission: November 18, 2023;Published: January 23, 2023
*Corresponding author: Agele Samuel O, Department of Biology, Federal University of Technology, Akure, Nigeria
How to cite this article: Ogunwole Ayodeji A, Agele Samuel O, Ologundudu F. Effects of Watering Regime and Biochar on the Growth, Biochemical Constituents and Drought Susceptibility of Seedlings of Some Urban Forest Tree Species. JOJ Hortic Arboric. 2023; 3(4): 555621. D. DOI 10.19080/JOJHA.2023.03.555621.
Abstract
Background: Climate stress and soil constraints of urban landscapes would limit establishment, shade effectiveness and ecosystem services of urban forests. Developments of the plant components of urban green infrastructure must identify tree species that are well adapted to climate stress factors. The effects of effects of wet-dry cycle and biochar on the growth and physiological attributes, biochemical constituents, and drought susceptibility of seedlings of some urban forest tree species (UFTS) was evaluated in the nursery.
Methods: The tested UFTS were Bauhinia monandra, Delonix regia, Terminalia catappa, Dypsis lutescens and Veitchia merrillii. Seedlings were subjected to 80 % field capacity (FC) watering and dry down treatment, and biochar amendment or not. Measured growth physiological and biochemical variables of UFTS seedlings were deployed for ranking the evaluated UFTS for drought susceptibility.
Results: Relative to well watering, dry down treatment produced lower values of root and shoot biomass, leaf area, relative water content, net assimilation rates, leaf chlorophyll and carotenoids contents. Dry down treatment enhanced accumulation of proline, soluble sugars, flavonoids and crude protein contents and elicited changes in enzymatic activities. Biochar amendment of dry down treatment enhanced growth, physiological and biochemical attributes of UFTS seedlings and induced enzymatic antioxidant activities. The increasing trends of drought tolerance among the UFTS were B. monandra, D. regia, T. catappa, D. lutescens and V. merrillii for both biochar amended and non-amended dry down condition.
Conclusion: Study identified drought susceptibility of evaluated UFTS seedlings and relevance of biochar for moisture deficit stress alleviation.
Keywords: Urban Green Infrastructure; Antioxidant; Osmotic Adjustment; Enzymatic Activites; Moisture Deficit; Amendment
Abbreviations: ROS: Reactive Oxygen Species; UFTS: Urban Forest Tree Species; DSI: Drought Susceptibility Indices; HSD: Honest Significant Difference; TSS: Total Soluble Sugar; ASC: Ascorbic Acid; DSI: Drought susceptibility index
Introduction
Global environments change manifests as climate change and extreme weather events. Climate change may increase pressures on water resources, water stress situation and high temperatures with attendant shifts in environment boundaries [1,2]. Extreme temperatures will result in lethal heatwaves and urban heat island (UHI) phenomenon with consequences for human thermal comfort and heat related illnesses across the world. It is important to promote development of urban forestry into urban landscape development for building resilience to climate stress Roll of et al., 2009 [3,4]. Benefits of urban vegetation (urban green infrastructure) are attributable to tree modulation of solar radiation properties, evaporative cooling, carbon sequestration, water cycling and biodiversity conservation [5-7]. Climate stress and soil constraints of urban landscapes affect phenology and physiological functions, shade effectiveness, and ecosystem services of urban park and street trees [8,3,7]. In order to optimize the benefits of urban forestry, it is necessary to identify adaptable tree species to climate stress now and under future climate scenarios.
Strategies for abiotic stress tolerance in plants may involve complex, interacting mechanisms of. desiccation tolerance and drought performance [9,10] Mannan et al. 2016, [11]. In plants, water deficit stress may alter metabolic pathways, morphological, physiological, ultra-structural, biochemical features [12] Zhang et al. 2013, [13,14]. The effects of drought on msorphological and physiological traits and compatible metabolites and osmolytes of annual and perennial species has been evaluated severally [15- 17]. Limitations in soil moisture status affect phytochemicals such as total soluble solids, organic acids, and soluble carbohydrates Keller & Ludlow, 1993, Khan et al., 2015, [18-20] and Soni et al. (2015). Changes in chlorophyll and carotenoid contents have been implicated as index of plant response to drought stress Pastori and Trippi, 1992, [21], and functions to protect against damage to chloroplasts caused by active oxygen species [22,23]. Increases in proline accumulation in leaves of water-stressed plants have been reported [24,23]. This is attributable to proline’s role as a source of respiratory energy in addition to changes in the concept of free proline and total soluble sugars in water-stressed leaves [25,26].
Water stress is known to trigger excitation of photosynthetic pigments leading to accumulation of reactive oxygen species (ROS) such as singlet oxygen (O), superoxide anions (-O2-), peroxide (-O2-2), hydroxyl ion (HO-) and hydroxyl radical (OH⋅) in the chloroplasts. Reactive oxygen species can damage phospholipids of cell membrane and increase lipid peroxidation measured as Malondialdehyde (MDA) Mittler, 2002; Moller et al., 2007, [27]. In curtailing the impacts of oxidative stress, plants have developed complex enzymatic system involving reactive oxygen species (ROS), scavenging enzymes (superoxide dismutase, catalase; ascorbate peroxidase, Glutathione reductase etc.) and nonenzymatic antioxidants (glutathione, ascorbic acid, carotenoids) [28-30]. Urban landscapes exhibit specific microclimate and soil characteristics different from undisturbed ecosystems Degaetano, 2000, Rollof et al., 2009, [5].
Organic amendment, in particular, the use of biochar has been suggested as a viable tool to improve productivity, aesthetic performance, and carbon sequestration of urban forest species [31-34]. Biochar is a fine-grained and porous substance generated via pyrolysis of biomass-derived feed stocks under oxygen-limited conditions [35-36]. Biochar impacts soil properties: hydraulics, microbial activities, decomposition of native organic carbon content [38,35,39]. Soil amendment using biochar is considered as a means to mitigate impacts of water deficits on plants and for enhancing water use efficiency (Liang et al., considered as al., 2010, Eyles et al., 2015) [40-42]. The aim of this study is to evaluate the effects of watering regime and biochar amendment on the physiological attributes, biochemical constituents of seedlings of some urban forest tree species (UFTS) in the nursery.
Materials and Methods
The study was carried out in the screenhouse of Wesley University, Ondo, Nigeria (Geo-coordinates of: 7.100005, 4.841694; DMS Lat. 7º6ʹ0.0180ʹʹN; DMS Long. 4º50ʹ30.0984ʹʹE, 387 m above sea level in a rainforest zone of southern Nigeria. Seedlings of urban forest trees species namely, Bauhinia monandra Kurz (Fabaceae), Delonix regia (Bojer ex Hook) Raffin (Fabaceae), Terminalia catappa L. (Combretaceae), Dypsis lutescens (H. Wendl.) Beentze & J. Dransf. (Arecaceae) and Veitchia merrillii (Becc.) H. E. Moore (Arecaceae) were raised by sowing seeds in plastic pots (Upper × Lower diameter × Height = 26 × 20 × 30 cm) containing 6 kg of top garden soil. Seedlings were watered to pot capacity for one month to allow acclimatization and thereafter subjected to well watering (80 % field capacity: FC) and dry down treatements, and sawdust biochar amendment or not. Dry down treatment was imposed by water withholding from potted UFTS seedlings for 42 days (6 weeks) followed by re-watering after treatment had induced significant amount of leaf abscission. The measured UFTS variables were deployed to compute species drought susceptibility indices (DSI) in order to rank the aesthetic performance and species tolerance of soil moisture deficit stress. For each species and treatment, 30 pots with one seedling each were maintained. Measurement of morphological and physiological traits and biochemical constituents were carried out monthly and average of the pooled data were subjected to statistical analysis for comparison of growth attributes and drought susceptibility of each tested species. Stem height and girth, number of leaves per plant, root length and ratio of root to shoot were measured using standard methods. Plant leaf area was determined using Pearcy et al., (1989) and total leaf area was estimated as the product of leaf area and number of leaves per plant. Biomass (dried at 80 ± 2°C) of leaf, stem and roots was recorded by harvesting three seedlings from each treatment. Photosynthetic pigment (chlorophyll a, b and total chlorophyll and carotenoids) and relative water contents were estimated using Weatherley (1950) and Lichtenthaller (1987) formulas respectively.
Estimation of Biochemical Constituents and Enzymatic antioxidants activities in UFTS
Contents of proline, soluble sugar (TSS), ascorbic acid (Asc) and phenolic acid (TPC) on fresh weight basis were estimated according to the procedures of [43,44] AOAC (2000) and [45] respectively. Peroxidation of membranes lipid measured as malondialdehyde (MDA) content was determined by following the methods of Hodges et al., (1999). For assessment of activities of superoxide dismutase (SOD), catalase (CAT) and guaiacol peroxidase (GPx), the procedures of Giannopolitis and Ries (1977), Aebi and Lester (1984) and Chance and Maehly (1955) were adopted.
Drought Susceptibility of UFTS Seedlings
The drought susceptibility index (DSI) measured in percentages was calculated as the ratio of some measured morphological, physiological, and biochemical attributes of urban tree species under well-watered (X2) and water stress conditions (X1) as.
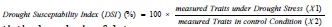
Statistical analysis of data
Data obtained on measured variables of the UFTS seedlings were subjected to two way analysis of variance (ANOVA) while the significant means was separated using the Tukey’s Honest Significant Difference (HSD) test at P < 0.05.
Results
The treatments (well watering and dry down treatments and biochar application) affected the growth, physiology and biochemical constituents and drought susceptibility of seedlings of tested urban forest species. Among the seedlings, differences were observed between well watering and dry down condition for stem height, leaf area, root and stem and leaf biomass. Differences observed were significant for number of leaves and shoot weights between well watering and dry down treatments (Table 1). Biochar amendment of dry down treatment enhanced almost all growth variables of UFTS seedlings as well as physiological attributes and biochemical constituents of seedlings of urban forest species. For both biochar amended and non-amended dry down treatments, leaf relative water content (RWC) of B. monandra, D. regia, T. catappa, D. lutescens and V. merrillii seedlings substantially (P<.05) reduced compared to values under well watering (Table 2). The inclusion of biochar to dry down induced increases of ~ 17.0, 2.4, 10.8, 7.8 and 5.3 % in leaf RWC of seedlings respectively compared with dry down without biochar amendment.
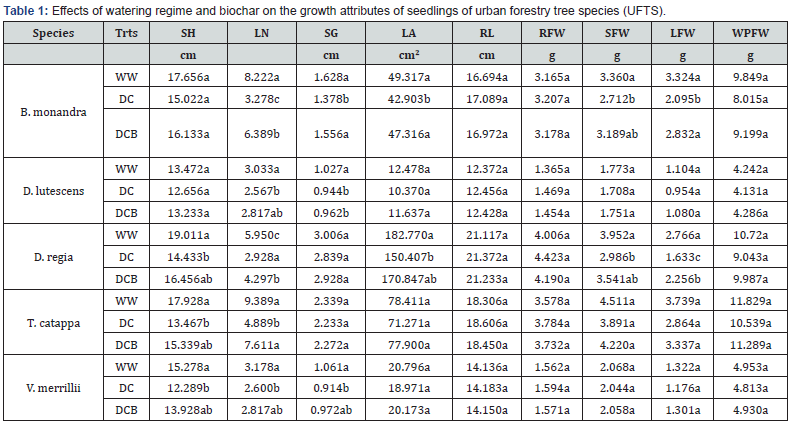
Values along the columns bearing same letters are not significantly different (α = 0.05). WW denotes adequate watering (at 80 %FC), DC denotes dry down, DCB denotes dry down plus biochar. Shoot height (SH), number of leaves (LN), stem girth (SG), leaf area (LA), total leaf area (TLA), root length (RL), root fresh weight (RFW), stem fresh weight (SFW), leaf fresh weight (LFW), whole plant fresh weight (WPFW).
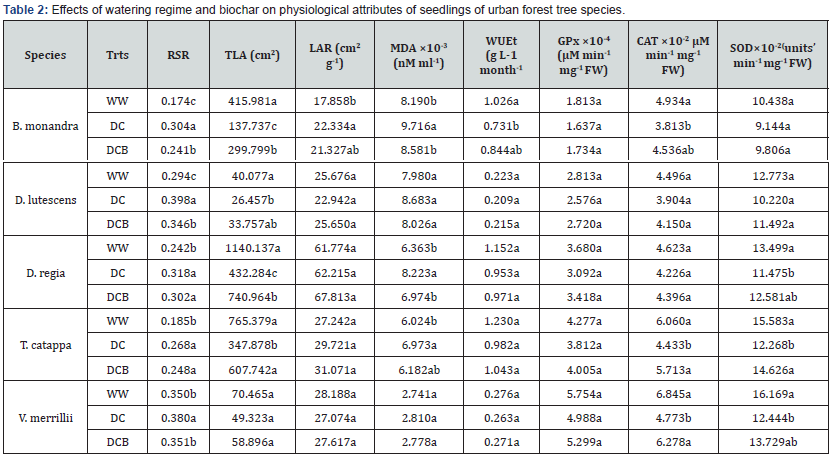
Values along the columns bearing same letters are not significantly different (α = 0.05). Root shoot ratio (RSR), total leaf area (TLA), leaf area ratio (LAR), water use efficiency (WUEt), malondialdehyde content (MDA), guaiacol peroxidase (GPx), catalase (CAT), and superoxide dismutase (SOD).
The effect of dry down was profound on malondialdehyde (MDA) content of seedlings (Table 2). Dry down significantly (P<.05) increased MDA content by 18.6, 29.2 and 15.8% in B. monandra, D. regia and T. catappa seedlings respectively, relative to values under well watering and no such increase was found for other UFTS seedlings. Activities of antioxidant enzymes: superoxide dismutase (SOD), guaiacol peroxidase (GPx) and catalase (CAT) differed among the UFTS. The lowest activities were found for seedlings under dry down conditions (Table 2). Under dry down treatment, activities of SOD and CAT declined significantly (P<.05) in B. monandra seedlings (~ 21.3 and 26.8 % respectively) and in D. lutescens seedlings (30.6 and 41.7%). Superoxide dismutase activity significantly (P<.05) reduced by 15.0% in T. catappa seedlings while activity of CAT in D. regia seedlings significantly (P<.05) reduced by 28.9% compared with activities for well-watered seedlings. Under dry down also decreased the activities of SOD and GPx among seedlings. Biochar amendment of dry down condition appear to ameliorate the negative effects of high soil water deficit (dry down condition) such that antioxidant enzymes activities of SOD, CAT and GPx were statistically similar to seedlings under adequately watering (Table 2).
Generally, decreases in antioxidant enzyme ativities under un-amended dry down treatment follow the order: D. lutescens > B. monandra > D. regia > T. catappa > V. merrillii. Biochar amendment of dry down decreased SOD and CAT activities of seedlings in decreasing order of: D. lutescens > D. regia > T. catappa > B. monandra > V. merrillii and increase GPx activity in the order: B. monandra > D. lutescens > D. regia > T. catappa > V. merrillii. The chlorophyll contents of UFTS seedlings decreased under dry down condition. The contents of chlorophyll a, chlorophyll b and total chlorophyll were significantly lowered by ~ 46.3, ~ 49.4 and ~ 47.7% in B. monandra; 38.9, 41.6 and 40.1% in D. regia; 17.6, 27.3 and 22.0% in T. catappa, 32.7, 38.8 and 35.5% in D. lutescens; and 31.0, 33.3 and 32.1% in V. merrillii respectively (Table 3). The contents of light capturing pigments decreased significantly (P<.05) by 27.9, 32.5 and 30.0% respectively in D. lutescens and by 34.6, 39.3 %, 36.8% in D. regia and and 30 % in V. merrillii seedlings seedlings upon biochar amendment of dry down condition. Dry down treatment without biochar amendment decreased chlorophyll a concentration in the following order: D. regia > B. monandra > D. lutescens > V. merrillii > T. catappa while the trends of chlorophyll b content followed the order: D. regia > D. lutescens > B. monandra > V. merrillii > T. catappa (Table 3). Under dry down, decreasing Car/Chl ratio is: V. merrillii > B. monandra > D. regia > D. lutescens > T. catappa. While biochar amendment increased Chl. a/b as follows: T. catappa > D. lutescens > D. regia > V. merrillii > B. monandra.
Dry down treatment without biochar amendment enhanced accumulation of proline, TSS, Asc and TPC (Table 3). Accumulation of proline, ASC and TPC significantly (P<.05) increased by ~ 34.1, 12.5 and 14.1% respectively in V. merrillii seedlings, TSS and ASC contents remarkably (P<.05) increased by ~ 19.0 and 22.7% for B. monandra seedlingorder of s while only ASC content increased considerably (P<.05) by ~ 17.4% in D. lutescens seedlings (Table 3). The highest increment of carotenoids 45.3% for D. regia (45.3%) under dry down treatment without biochar treatment while the lowest increment (3.9%) was detected in V. merrillii seedlings under biochar-dry down condition (Table 3). Relative to their contents under well watering, dry down condition increased the concentrations of proline, total soluble sugar (TSS), ascorbic acid (ASC) and total phenolic acid (TPC) in D. regia seedlings by about 30.8, 21.6, 13.5 and 30.4% while the contents of these biomolecules spiked by ~ 21.0, 14.1, 28.0 and 18.6% in T. catappa seedlings.
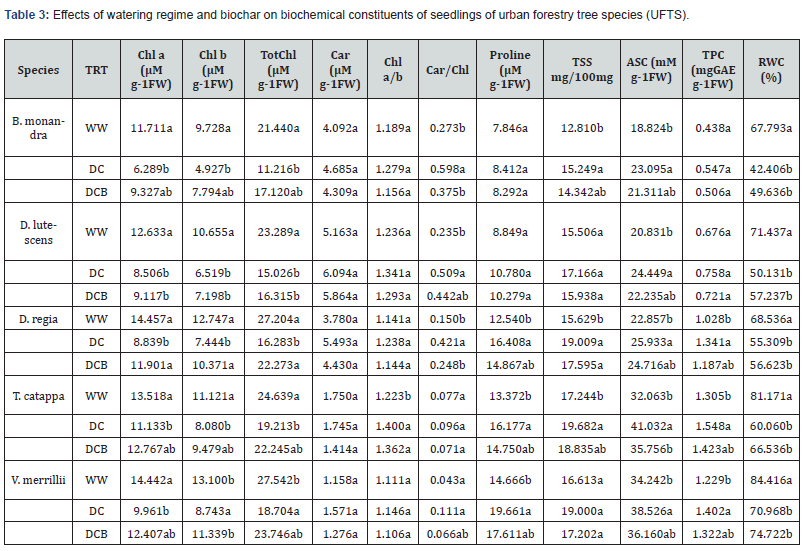
Values along the columns bearing same letters are not significantly different (α = 0.05). Relative water content (RWC), chlorophyll a content (Chl.A), chlorophyll b content (Chl. b), total chlorophyll content (TotChl), ratio of chlorophyll a to chlorophyll b (Chl a/b), ratio of carotenoids to total chlorophyll content (Car/Chl), total soluble sugar (TSS), ascorbic acid content (ASC.), total phenolic acid content (TPC).
Among UFTS, increment in TSS follows the trend of: B. monandra > D. regia > T. catappa > V. merrillii > D. lutescens. The increase in accumulated proline follows the order; V. merrillii > D. regia > D. lutescens > T. catappa > B. monandra, the increment in Asc content was of the oder: B. monandra > T. catappa > D. regia > D. lutescens > V. merrillii whereas increment in TPC was of the order: B. monandra > D. regia >T. catappa > V. merrillii > D. lutescens. However, biochar amendment of dry down treatment increased the accumulation of proline, TSS, ASC and TPC in the UFTS. Proline accumulation among UFTS follows the trend: V. merrillii > D. regia > T. catappa > D. lutescens > B. monandra while that of TSS was of the order; V. merrillii > B. monandra > D. regia > T. catappa > D. lutescens. Further, ASC content of biochar-amended dry down followed the order; T. catappa > B. monandra > D. lutescens > D. regia > V. merrillii whereas TPC was of the trend; D. regia > B. monandra > T. catappa > V. merrillii > D. lutescens (Table 3).
Drought susceptibility and aesthetic performance of UFTS
Drought susceptibility index (DSI) values were deployed to determine the effect of biochar and watering regime on shoot height of the UFTS seedlings (Table 4). Results indicated decreasing order of shoot height among the species as: D. regia > T. catappa > V. merrillii > B. monandra > D. lutescens. Effects of dry down on seedling leaf area in decreasing trend of: D. regia > D. lutescens > B. monandra > T. catappa > V. merrillii while DSI values of plant leaf area follows the order; B. monandra > D. regia > T. catappa > D. lutescens > V. merrillii. Biochar susceptibility index (BSI) showed biochar enhancement of plant leaf area in decreasing order of: D. regia > D. lutescens > B. monandra > V. merrillii > T. catappa. The increment in carotenoids contents in seedlings under dry down condition followed the trend V. merrillii > D. regia > T. catappa > D. lutescens > B. monandra. Biochar susceptibility index values showed decreasing order of: V. merrillii > D. lutescens > D. regia > T. catappa > B. monandra. Dry down significant (P<.05) enhanced root: shoot ratio by: ~ 74.7, 37.7, 53.1, 25.5 and 13.1% for Bauhinia, Delonix, Terminalia, Dypsis and Veitchia seedlings. Upon inclusion of biochar, root:shoot of B. monandra, D. regia, T. catappa and D. lutescens remarkably (P<.05) increased by ~ 38.5, 30.1, 41.7and 15.3% (Table 4). The ratio of carotenoids to total chlorophyll content (Car/Chl) of seedlings was high under dry down compared with well watering treatment.
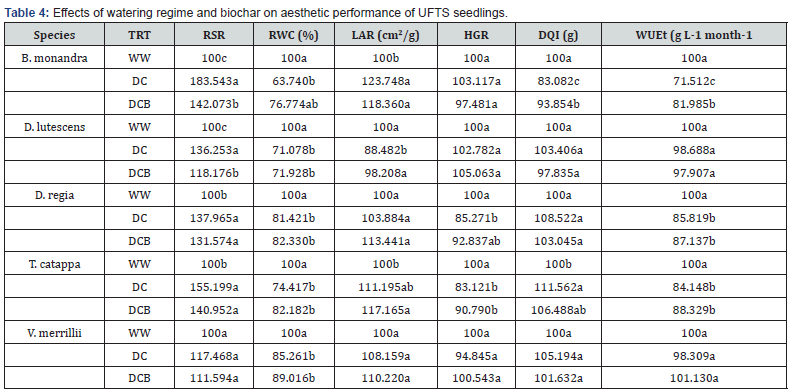
Values along the columns bearing same letters are not significantly different (α = 0.05). Root shoot ratio (RSR), relative water content (RWC), leaf area ratio (LAR), ratio of shoot height to stem girth (HGR), Dickson quality index (DQI), water use efficiency (WUEt), guaiacol peroxidase (GPx), catalase (CAT), and superoxide dismutase (SOD).
Non biochar-amanded dry down significant (P<.05) decreased Car/Chl ratios by ~ 119.0, 180.7, 116.6 and 122.0% for B. monandra, D. regia, D. lutescens and V. merrillii seedlings compared with biochar amendmentand well-watered seedlings. The DSI values of plant leaf area followed the order; B. monandra > D. regia > T. catappa > D. lutescens > V. merrillii. Under dry down condition, total plant biomass followed decreasing order of: B. monandra > T. catappa > D. regia > V. merrillii > D. lutescens. Following biochar amendment, drought susceptibility shows decreasing trends of total plant biomass among UFTS as: B. monandra > D. regia > T. catappa > V. merrillii > D. lutescens for WPDW and B. monandra > D. regia > T. catappa > V. merrillii > D. lutescens (Table 4). Increment in carotenoids contents of seedlings under dry down followed the trend V. merrillii > D. regia > T. catappa > D. lutescens > B. monandra. Drought susceptibility index showed reduction in relative water content of leaves among UFTS seedlings in both the non-amended and biochar amended dry down condition in the order of: V. merrillii < D. regia < T. catappa < B. monandra < D. lutescens.
Discussion
Among the UFTS evaluated, differences were obtained in growth, physiology, and biochemical constituents under well watering and dry down conditions with or without biochar ammendment. The significant decrease in leaf production by D. regia, T. catappa and especially B. monamdra seedlings could have resulted from deciduous habit of these species under high soil moisture deficit condition. This observation may constitute drought avoidance strategies in these species. The remarkable low number of fronds, shoot height and stem girth of V. merrillii and D. lutescens seedlings under extreme water deficit stress is similar to the reported findings on morphometrics of oil palm seedlings under the same condition [46,47]. Previous studies had observed a similar reduction in the leaf RWC of olive trees [48] under soil water deficit. The low chlorophyll contents of seedlings under dry down conditions is consistent with the findings of Silva- Pinheiro et al. (2016) on B. monandra and Soliman et al. (2015) on D. regia. Such decreases in chlorophyll contents could be related to elevated ROS formation induced by water deficit stress with consequences on photosynthesis and carbon assimilation [49,50]. The relatively high chlorophyll content in V. merrillii and D. regia seedlings under dry down condition could indicate species drought tolerance ability or inherently rich in chlorophyll pigment. The lack of significant impact of drought on carotenoids content of UFTS seedlings probably suggests other photosystem protective mechanisms other than carotenoids. These species might have sunscreen their photosynthetic apparatus by increasing osmolytes contents or enhanced activities of SOD, CAT and GPx or APx. Kesharvarz-Afshar et al. (2016) reported similar findings on Silybum marianum.
The differences observed in osmolyte concnetrations (proline, total soluble sugar, ascorbic acid, phenolic acid and total flavonoids) among seedlings indicate species-specificity of these traits. Changes in osmolytes concnetrations have been reported as a strategy to maintain osmotic potential (Aranjuelo et al., 2010) [23] and enhancing oxidative stress tolerance [51]. Generally, there was high ascorbic acid content (Asc) among the UFTS seedlings under dry down condition which may confirm that mesic UFTS seedlings enrouted the ascorbic acid peroxidase (APx) pathway to combat the drought-induced oxidative stress. Osmolytes, especially ascorbic acid supply electrons for the activation of antioxidant enzymes especially APx for scavenging ROS causing dismutation of H2O2 to water and molecular oxygen under water deficit stress [52, 53]. The significantly higher content of proline and total soluble sugar (TSS) in B. monandra seedlings and greater accumulation of proline, TSS and total phenolic acid (TPC) in T. catappa and D. regia seedlings under soil water deficit could indicate drought tolerance strategy in the species [25, 26]. The contents of proline, soluble sugar and phenolic acid appeared as plausible ecological markers for monitoring drought stress. This result agrees with the findings of [25,26,54] on D. regia. Proline and TPC contents were higher under dry down for V. merrillii seedlings compared with D. lutescens. Similar increase in the proline content had been reported in oil palm [55,56] and coconut palm [35]. [57,35] obtained low accumulation of proline in coconut under moisture stress for water balance regulation.
In this study, increases in contents of osmolytes were found in seedling leaves under biochar –amendment of dry down soil. This implies that as soil dries out, water is released from micro- and meso-pores of biochar amended soil resulting in low production of ROS and increased activities of enzymatic antioxidants. Biochar contains high contents of cations which may promote increased accumulation of osmotic active substances such as K+ in the plant tissues, resulting in an improved water uptake (Gaskin et al., 2010) and reduced quantity of ROS formation. The significant increase in MDA content of B. monandra, T. catappa and D. regia seedlings under dry down condition may be related to chloroplast membrane function. However, the dehydration of cytoplasm would have resulted in damage to photosynthetic apparatus due to higher levels of accumulated ROS. Under extreme water deficit stress condition, remarkable increase in the MDA content had also been observed in Eucalyptus globulus [58], Jathropha curcas [59]. In this study, V. merrillii and D. lutescens was least affected by lipid peroxidation due to low level of accumulated MDA under dry down condition. Thus, V. merrillii seedlings specifically exhibited relatively higher drought tolerance than semi deciduous species and such observation may be attributed to efficient regulation of stomata movement and enzymatic antioxidants activities. Plants increase the activities of antioxidant enzymes (CAT, SOD, APx and manage ROS accumulation under drought [60]. Increased activity of enzymatic antioxidants under stress conditions for protection from oxidative damage [61,62]. However, dry down treatment suppressed the activities of CAT and SOD in B. monandra and D. lutescens seedlings revealing higher susceptibility of species to oxidative stress.
Drought susceptibility of tested UFTS seedlings (using drought susceptibility indices)
Observations on the drought susceptibility (using drought susceptibility indices: DSI) showed that the UFTS seedlings differed in susceptibility to soil moisture deficit stress. Higher susceptibility of B. monandra than D. regia seedlings were consistent with the results of Hassanein (2015) on these species. The activities of SOD in T. catappa and CAT in D. regia declined significantly under dry down condition (more drought susceptible species) compared with V. merrillii seedlings which upregulated enzymatic activities (SOD, CAT and GPx) under moisture deficit. The UFTS evaluated tended to survive water deficit stress using avoidance mechanism by allocating more carbon resources to the root relative to shoots for enhancement of water extraction. In particular, B. monandra, D. regia, T. catappa and V. merrillii seedlings had reduced aboveground biomass compared with root biomass while D. lutescence seedlings tended to favour the shoot system compared with root in carbon allocation.
Contrary to the decrease in the chlorophyll a/b ratio reported for several agricultural crops [62,63]. The Chl a/b ratio increased significantly in T. catappa seedlings and slightly in other UFTS under water deficit which depicts the differential response of chlorophyll synthesis and degradation between agricultural crops and trees under drought. A similar increase in the Chl a/b of date palm was reported by Shareef et al. (2020). The biosynthesis/ degradation of photosynthetic pigments is relevant as plant stress tolerance mechanism underlying abiotic stress [64,65]. Leaf carotenoids content (LCar) is an important indicator of plant physiological status and are known to shield the photosynthetic apparatus against photo-oxidation by quenching ROS, particularly singlet oxygen under extreme condition of water stress (Caretto et al,. 2002), [66]. Under high soil moisture deficit stress, decreases in Car/Chl ratio follow the trend: Veitchia > Bauhinia > Delonix > Dypsis > Terminalia. This observation may indicate the susceptibility of chloroplast of UFTS seedlings to photo-oxidation under drought.
Biochar-enhancement of UFTS tolerance of soil moisture deficit
Biochar amendment of dry down treatment improved growth traits of T. catappa; and V. merrillii seedlings under dry down condition significantly. Such improvements could have stemmed from biochar improvement of soil properties [35, 67-69] and that drought mitigating capacity of biochar is species-specific [67,68]. Addition of biochar to dry down treatment could not effectively mitigate the negative impact of moisture stress as eflected in statistically lower leaf relative water content of UFTS seedlings under these treatments. This may indicate the existence of a threshold for biochar efficiency when adopted for drought alleviation in plants. Biochar amendment of dry down treatment maintained the levels of chlorophyll contents of B. monandra, D. regia, T. catappa and V. merrillii seedlings via reduction of droughtinduced oxidative stress and maintenance of metabolic processes and increased activity of anti-oxidant enzymes [42,70]. Previous studies reported positive influence of biochar on water stressed Eucalyptus globulus (Amaral, 2014), Phragmites karka [71] sand barley [60]. The results were ascribed to biochar-enhanced reduced oxidative stress, lipid peroxidation and enzymatic antioxidants. Our results establish the differentials response of seedlings to biochar application in particular under dry down condition for improved aesthetic values of UFTS. In addition to the drought mitigating potential of biochar is its relevance for conditioning urban soil for nursery production for improving growth and aesthetic value of UFTS [73-88].
Conclusion
Treatment (well watering, dry down treatment and biochar amendment or not) effects was profound on the growth and physiological attributes, biochemical constituents and drought susceptibility of seedlings of urban forest species evaluated. Seedling attributes (root and shoot biomass, relative water content, plant leaf area, relative growth and net assimilation rates total leaf chlorophyll and carotenoids contents, ratios of chlorophyll to carotenoids) were significantly lower under dry down treatment relative to well watering. Dry down treatment elicited changes in growth and physiological attributes and biochemical constituents of seedlings of the evaluated UFTS. Dry down treatment enhanced the accumulation of osmolytes (proline, total soluble sugar (TSS), total flavonoids (TFC) and crude protein contents) and elicited changes in enzymatic activities (reduced Superoxide Dismutase Catalase activities and increased Guaiacol Peroxidase and Malondialdehyde Contents of seedlings.
Biochar amendment of dry down treatment enhanced values of growth, physiological and biochemical attributes of UFTS seedlings and modified enzymatic antioxidant activities. Biochar amendment appears to mitigate the negative effects of moisture deficit stress (dry down treatment) promoted accumulation of osmolyte (proline, TSS, ASC and TPC) and reduced enzymatic antioxidant activities (SOD, CAT and GPx. The strategies for improved drought tolerance exhibited by UFTS seedlings include improvement of antioxidant capacity, osmotic adjustment capacity (accumulation of osmolytes for protection of photosynthetic system) and reduced enzymatic antioxidant activities (SOD and CAT). The drought mitigating capacity of UFTS using sawdust biochar was affirmed. Study confirmed the drought susceptibility of the evaluated UFTS seedlings. Increasing order of drought tolerance of UFTS were B. monandra, D. regia, T. catappa, D. lutescens and V. merrillii.
References
- Shigeoka S, Ishikawa T, Tamoi M, Miyagawa Y, Takeda T, et al. (2002) Regulation and function of ascorbate peroxidase isoenzymes. Journal of Experimental Botany 53(372): 1305-1319.
- Trenberth KE, Dai A, Schrier van der G, Jones PD, Barichivich J, et al. (2014) Global warming and changes in drought. Nature & Climate Change 4: 17-22.
- Agele S (2021) Global Warming and Drought, Agriculture, Water Resources, and Food Security: Impacts and Responses from the Tropics.
- Ordonez C, Duinker PN (2014) Assessing the vulnerability of urban forests to climate change. Environmental Reviews 22(3): 311-321.
- Rahman MA, Armson D, Ennos AR (2015) A comparison of the growth and cooling effectiveness of five commonly planted urban tree species. Urban Ecosystem 18: 371-389.
- Cameron RWF, Harrison-Murray RS, Atkinson CJ, Judd HL (2006) Regulated deficit irrigation: A means to control growth in woody ornamentals. Journal of Horticultural Science and Biotechnology 81(3): 435-443.
- Doughty CE, Roman J, Faurby S, Wolfe A, Haque A, et al. (2015) Global nutrient transport in a world of giant. Proceedings of the National Academy of Sciences 113(4): 868-873.
- Rahman M A, Moser A, Rotzer T, Pauleit S (2017) Microclimatic differences and their influence on transpirational cooling of Tilia cordata in two contrasting street canyons in Munich, Germany. Agricultural and Forest Meteorology 232: 443-456.
- Zollinger N, Kjelgren R, Cerny-Koenig T, Kopp K, Koenig R (2006) Drought responses of six ornamental herbaceous perennials. Scientia Horticulturae 109(3): 267-274.
- Tyree MT, Engelbrecht BMJ, Vargas G, Kursar TA (2003) Desiccation Tolerance of Five Tropical Seedlings in Panama. Relationship to a Field Assessment of Drought Performance. Plant Physiology 132(3): 1439-1447.
- Tombesia S, Frionia T, Ponia S, Palliotti A (2018) Effect of water stress “memory” on plant behavior during subsequent drought stress. Environmental & Experimental Botany 150: 106-114.
- Stewart CR (1981) Proline accumulation: Biochemical aspects. Physiology and Biochemistry of drought resistance in plants (Paleg, L.G. &Aspinall D. eds) pp. 243-251.
- Watkins JM, Chapman JM, Muday GK (2017) Abscisic acid-induced reactive oxygen species are modulated by flavonols to control stomata aperture. Plant Physiology 175(4): 1807-1825.
- Li X, Liu F (2016) Drought stress Memory and Drought stress tolerance in plants: biochemical and molecular basis. Drought Stress Tolerance in Plants 1 (Hossain, M.A. Ed.). Springer International, Switzerland 1: 17-44.
- Hsiao TC, Acevedo E, Fereres E, Henderson DW (1976) Water stress, growth, and osmotic adjustment. Phil. Trans. Roy. Soc. Lond. B 273(927): 479-500.
- Tezara W, Mitchell VJ, Driscoll S, Lawlor DW (1999) Water stress inhibits plant photosynthesis by decreasing coupling factor and ATP Nature 401: 914-917.
- Glenn DM, Kim SH, Ramirez-Villegas J, Laderach P (2014) Response of perennial Horticultural crops to climate change. Horticultural Reviews 41: 47-130.
- Shivashankar S, Kasturi Bai K V, Rajagopal V (1991) Leaf water potential, stomatal resistance, and activity of enzymes during development of moisture stress in the coconut palm. Tropical Agriculture 68: 106-10.
- Mafakheri A, Siosemardeh A, Bahramnejad B, Struik PC (2011) Effect of drought stress and subsequent recovery on protein, carbohydrate contents, catalase, and peroxidase activities in three chickpeas (Cicer arietinum) cultivars. Australian Journal of Crop Science 5(10): 1255-60.
- Scalabrin E, Radaelli M, Rizzato G, Bogani P, Buiatti M, et al. (2015) Metabolomic analysis of wild and transgenic Nicotiana langsdorffii plants exposed to abiotic stresses: Unraveling metabolic responses. Anal Bioanal Chem 407: 6357-6368.
- Khayatnezha M, Gholamin R (2012) The effect of drought stress on leaf chlorophyll content and stress resistance in maize cultivars (Zea mays). African Journal of Microbiology Research 6(12): 2844-2848.
- Aranjuelo I, Molero G, Erice G, Avice JC, Nogués S (2011) Plant physiology and proteomics reveals the leaf response to drought in alfalfa (Medicago sativa L.). J Exp Botany 62(1): 111-123.
- Smirnoff N. (1995) Antioxidant systems and plant response to the environment. Environment and Plant Metabolism. Flexibility and Acclimation (Smirnoff, V. ed.). BIOS Scientific Publishers pp. 217-243.
- Raza MAS, Shahid AM, Saleem MF, Khan IH, AhmadS, et al. (2016) Effects and management strategies to mitigate drought stress in oilseed rape (Brassica napus L.): a review. Zemdirbyste 104(1): 85-94.
- Al-Hassan M, Lopez-Gresa MP, Boscaiu M, Vicente O (2016) Stress tolerance mechanisms in Juncus: responses to salinity and drought in three Juncus species adapted to different natural environments. Functional Plant Biology 43(10): 949-960.
- Akram NA, Jabeen M, Ashraf M, Aziz A (2019) Assessment of Biochemical Changes in Spinach (Spinacea oleracea L.) subjected to Varying Water Regimes. Sains Malaysiana 48(3): 533-541.
- Abedi T, Pakniyat H (2010) Antioxidant Enzyme Changes in Response to Drought Stress in Ten Cultivars of Oilseed Rape (Brassica napus L.). Czech Journal of Genetics and Plant Breeding 46: 27-34.
- Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annual Review of Plant Biology 55: 373-399.
- Ge Y, He X, Wang J, Jiang B, Ye R, et al. (2014) Physiological and biochemical responses of Phoebe bournei seedlings to water stress and recovery. Acta Physiological Plantarum (36): 1241-1250.
- Türkan Ý, Bor M, Özdemir F, Koca H (2005) Differential responses of lipid peroxidation and antioxidants in the leaves of drought - tolerant Phaseolus acutifolius Gray and drought-sensitive Phaseolus vulgaris L, subjected to polyethylene glycol mediated water stress. Plant Science 168(1): 223-231.
- Sohi S, Krull E, Lopez-Capel E, Bol R (2010) A review of biochar and its use and function in soil. Advances in Agronomy, 105: 47-82.
- Sarong M, Orge RF (2015) Effect of rice hull biochar on the fertility and nutrient holding capacity of sandy soils. OIDA International Journal of Sustainable Development 8(12): 33-44.
- Soliman AS, Morsy EM, Massoud ON (2015) Tolerance of bio fertilized Delonix regia seedlings to irrigation intervals. Journal of Horticulture and Forestry 7(3): 73-83.
- Haider I, Iqbal R, Aslam MU, Raja S, Khan MT, et al. (2020) Potential effects of biochar application on mitigating the drought stress implications on wheat (Triticum aestivum L.) under various growth stages. Journal of Saudi Chemical Society 24(12): 974-981.
- Lehmann J, Gaunt J, Rondon M (2006) Biochar sequestration in terrestrial ecosystems-A review. Mitigation and Adaptation Strategies for Global Change 11: 403-427.
- Kasturi Bai KV, Rajagopal V (2000) Osmotic adjustment as a mechanism for drought tolerance in coconut (Cocos nucifera L.). Indian Journal of Plant Physiology 5: 320-323.
- Masiello CA, Dugan B, Brewer CE, Spokas K, Novak JM, et al. (2014) Biochar effects on soil hydrology. In Biochar for Environmental Management Science, Technology and Implementation, eds 541-560.
- Wardle DA, Nilsson MC, Zackrisson O (2008) Fire-derived charcoal causes loss of forest humus. Science 320(5876): 629.
- Hansen V, Hauggaard-Nielsen H, Petersen CT, Mikkelsen TN, Müller-Stöver D (2016) Effects of gasification biochar on plant-available water capacity and plant growth in two contrasting soil types. Soil and Tillage Research 161: 1-9.
- Jeffery S, Verheijen FG, Van der Velde M, Bastos AC (2011) A quantitative review of the effects of biochar application to soils on crop productivity using meta-analysis. Agriculture, Ecosystems and Environment 144(1): 175-187.
- Basso AS, Miguez FE, Laird DA, Horton R, Westgate M (2013) Assessing potential of biochar for increasing water-holding capacity of sandy soils. Global Change Biology (GCB) Bioenergy 5: 132-143.
- Keshavarz-Afshar R, Hashemic BM, DaCosta M, Spargod J, Sadeghpoure A (2016) Biochar Application and Drought Stress Effects on Physiological Characteristics of Silybum marianum. Communications in Soil Science and Plant Analysis 47(6): 743-752.
- Bates CJ, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39: 205-207.
- Irigoyen JJ, Emerich DW, Sanchez Diaz M (1992) Water stress induced changes in concentrations of proline and total soluble sugars in nodulated alfafa (Medicago sativa) plants. Physiologia Plantarum 84(1): 55-60.
- Ainsworth EA and Gillespie KM (2007) Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nature Protocols, 2(4): 875-877.
- Suresh K, Nagamani C, Ramachandrudu K, Mathur RK (2010) Gas-exchange characteristics, leaf water potential and chlorophyll a fluorescence in oil palm (Elaeis guineensis Jacq.) seedlings under water stress and recovery. Photosynthetica 48: 430-436.
- Ibrahim MH, Najihah TS, Razak AA, Nulit R, Wahab PEM (2019) Effects of water stress on the growth, physiology and biochemical properties of oil palm seedlings. Agriculture and Food 4(4): 854-868.
- Trabelsi L, Gargouri K, Hassena AB, Mbadra C, Ghrab M, et al. (2019) Impact of drought and salinity on olive water status and physiological performance in an arid climate. Agricultural Water Management 213: 749-759.
- Ahmed F, Arthur E, Plauborg F and Andersen MN (2016) Biochar Effects on Maize Physiology and Water Capacity of Sandy Subsoil, Mechanization in Agriculture and Conserving of the Resources 6: 46-51.
- Aslam MU, Raza M A S, Saleem MF, Waqas W, Iqbal R, et al. (2020) Improving strategic growth stage-based drought tolerance in quinoa by rhizobacterial inoculation. Communication in Soil Science and Plant Analysis 51(7): 853-68.
- Akram NA, Waseem M, Ameen R, Ashraf M (2016) Trehalose pretreatment induces drought tolerance in radish (Raphanus sativus L.) plants: Some key physio-biochemical traits. Acta Physiologia Plantrum 38: 3.
- Mittler R, Vanderauwera S, Gollery M, Van Breusegem F (2004) Reactive oxygen gene network of plants. Trends in plant science, 9(10): 490-498.
- van Doorn W, Ketsa S (2014) Cross reactivity between ascorbate peroxidase and phenol (guaiacol) peroxidase. Postharvest Biology and Technology 95: 64-69.
- Sinhababu A, Banerjee A (2017) Selection of fuel wood yielding trees for agro forestry in dry lateritic area. Research Journal of Pharmaceutical, Biological and Chemical Sciences 8(4): 267-273.
- Cao HX, Sun CX, Shao HB, Lei XT (2011) Effects of low temperature and drought on the physiological and growth changes in oil palm seedlings. African Journal of Biotechnology 10(14): 2630-2637.
- Cha-um S, Yamadai N, Takabe T, Kirdmnee C (2013) Physiological features and growth characters of oil palm (Elaeis guineensis Jacq.) in response to reduced water-deficit and rewatering. Australian Journal of Crop Science 7(3): 432-439.
- Sean CC, Stefan KA, Janet EC, Sangeeta J, Narendra S, et al. (1998) The role of solute accumulation, osmotic adjustment, and changes in cell wall elasticity in drought tolerance in Ziziphus mauritiana (Lamk.). Journal of Experimental Botany 19(323): 967-977.
- Correia B, Pintó‐Marijuan M, Neves L, Brossa R, Dias MC, et al. (2014) Water stress and recovery in the performance of two Eucalyptus globulus clones: physiological and biochemical profiles. Physiol Plant 150(4): 580-592.
- Arcoverde GB, Rodrigues BM, Pompelli MF, Santos MG (2011) Water relations and some aspects of leaf metaboWater relationship curcas young plants under two water deficit levels and recovery. Brazilian Society of Plant Physiology 23(2): 123-130.
- Abdelaal K, Hafez Y, Attia K, Alamery S, Ghazy A, et al. (2020) Beneficial Effects of Biochar and Chitosan on Antioxidative Capacity, Osmolytes Accumulation, and Anatomical Characters of Water-Stressed Barley Plants. Agronomy 10(5): 630.
- Omidi H (2010) Changes of proline content and activity of antioxidative enzymes in two Canola genotype under drought stress. American Journal of Plant Physiology 5(6): 338-349.
- Ciscato M, Valcke R, Van Loven K, Clijsters H, Navari-Izzo F (1997) Effect of in vivo copper treatment on the photosynthetic apparatus of two Triticum durum cultivars with different stress sensitivity. Physiologia Plantarum 100(4): 901-908.
- Parida AK, Das AB, Mittra B (2003) Effects of NaCl stress on the structure, pigment complex composition and photosynthetic activity of mangrove Bruguiera parviflora chloroplasts. Photosynthetica 41:191-200.
- Al Omron AM, El-Maghraby SE, Nadeem MEA, El-Eter AM, Al-Mohani H (2012) Long term effect of irrigation with the treated sewage effluent on some soil properties of Al-Hassa Governorate, Saudi Arabia. Journal of the Saudi Society of Agricultural Sciences 11(1): 15-18.
- Shareef H, Abdi G, Fahad S (2020) Change in photosynthetic pigments of date palm offshoots under abiotic stress factors. Folia Oecologica 47(1): 45-51.
- Wang XC, Chen L, Ma CL, Yao MZ, Yang YJ (2010) Genotypic variation of beta-carotene and lutein contents in tea germplasms, Camellia sinensis (L.) O. Kuntze. Journal of Food Composition and Analysis 23(1): 9- 14.
- Manolikaki I, Diamadopoulos E (2019) Positive effects of biochar and biochar-compost on maize growth and nutrient availability in two agricultural soils. Communcation in Soil Science and Plant Analysis 50 (5): 512-526.
- Hogan MC (2011) “Respiration”. Encyclopedia of Earth.Eds. Mark McGinley and C. J. Clevel and. National Council for Science and the Environment. Washington DC.
- French E, Iyer-Pascuzzi AS (2018) A role for the gibberellin pathway in biochar-mediated growth promotion. Scientific Reports, 8: 1-10.
- Hou T, Berry TD, Singh S, Hughes M, Tong Y, et al. (2018) Control of tillage disturbance on the chemistry and proportion of raindrop-liberated particles from soil aggregates. Geoderma 330: 19-29.
- Abideen Z, Koyro HW, Huchzermeyer B, Ansari B, Zulfiqar F, et al. (2020) Ameliorating effects of biochar on photosynthetic efficiency and antioxidant defence of Phragmites karka under drought stress. Plant Biology 22(2): 259-266.
- Iqbal MD (2017) Utilization of biochar in improving yield of wheat in Bangladesh. Bulgarian Journal of Soil Science 2: 53-74.
- Agele S, Ajao M (2018) Responses of growth and yield of rice varieties to contrasting hydrothermal regimes during vegetative and reproductive growth phases in Akure, a rainforest zone of Nigeria. Intnational Journal of Plant & Soil Science 25 (5): 1-14.
- Arndt SK, Clifford SC, Wanek W, Joness HG, Popp, M (2001) Physiological and morphological adaptations of the fruit tree Ziziphus rotundifolia in response to progressive drought stress. Tree Physiology 21(11): 705-715.
- Arnold CL, Gibbons CJ (1996) Impervious surface coverage - The emergence of a key environmental indicator. Journal of the American Planning Association 62(2): 243-258.
- Boyer JS (1976) Photosynthesis of low water potentials. Phil. Trans. Roy. Soc. Lond 273(927): 501-512.
- Cha JS, Park SH, Jung SC, Ryu C, Jeon JK, et al. (2016) Production and utilization of biochar: A review. Journal of Industrial and Engineering Chemistry 40: 1-15.
- Garcia-Plazaola JI and Becerril JM (2000) Effects of drought on photoprotective mechanisms in European beech (Fagus sylvatica L) seedlings from different provenances. Trees -Structure and Function 14: 485-490.
- Giannopolitis CN and Ries SK (1977) Superoxide dismutase I. Occurrence in higher plants. Plant Physiology 59(2): 309-314.
- Gomes FP, Oliva MA, Mielke MS, Almeida AF, Aquino LA (2010) Osmotic adjustment, proline accumulation and cell membrane stability in leaves of Cocos nucifera submitted to drought stress. Scientia Horticulturae 126(3): 379-384.
- Haeberle KH, Agele SO, Matyssek R, Hennlich M (2016) Aspects of Water Relations and Gas Exchange of Katsura and Tilia Seedlings Subjected to Wet-Dry Cycles: Indication of Strategies for Whole Plant Drought Tolerance. International Journal of Soil & Plant Science 10(2): 1 -13.
- Haider G, Koyro HW, Azam F, Steffens D, Müller C, et al. (2015) Biochar but not humic acid product amendment affected maize yields via improving plant-soil moisture relations. Plant and Soil 395: 141-157.
- Kameyama K, Miyamoto T, Shiono T, Shinogi Y (2012) Influence of sugarcane bagasse-derived biochar application on nitrate leaching in calcaric dark red soil. Journal of Environmental Quality 41(4):1131-1137.
- Major J, Lehmann J, Rondon M, Goodale C (2010) Fate of soil-applied black carbon: downward migration, leaching and soil respiration. Global Change Biology 16(4): 1366-1379.
- Pietikainen J, Kiikkila O, Fritze H (2000) Charcoal as a habitat for microbes and its effect on the microbial community of the underlying humus. Oikos 89(2): 231-242.
- Raza MAS, Saleem MF, Khan IH, Jamil M, Ija M, et al. (2012) Evaluating the drought stress tolerance efficiency of wheat (Triticum aestivum L.) cultivars. Russian Journal of Agricuktural and Socio-Economical Sciences 12(12): 41-46.
- Soni P, Nutan KK, Soda N, Nongpiur RC, Roy S, et al. (2015) Towards Understanding Abiotic Stress Signaling in Plants: Convergence of Genomic, Transcriptomic, Proteomic, and Metabolomic Approaches. Elucidation Abiotic Stress Signal. Plants. 3 - 40.
- Wang WB, Kim YH, Lee HS, Kim KY, Deng XP, et al. (2009) Analysis of antioxidant enzyme activity during germination of alfalfa under salt and drought stresses. Plant Physiology and Biochemistry 47(7): 570-577.






























