Plant Tissue Culture in Two Varieties of Orthosiphon aristatus (Blume) Miq
Fahrauk Faramayuda1, Sukrasno1, Elfahmi1 and Totik Sri Mariani2*
1School of Pharmacy, Bandung Institute of Technology (ITB) & Faculty of Pharmacy Jenderal Achmad Yani University (UNJANI), Indonesia
2School of Life Sciences and Technology, Bandung Institute of Technology (ITB), Indonesia
Submission: September 09, 2019; Published: November 05, 2019
*Corresponding author: Totik Sri Mariani, School of Pharmacy, Bandung Institute of Technology (ITB) & Faculty of Pharmacy Jenderal Achmad Yani University (UNJANI), Indonesia
How to cite this article: Fahrauk Faramayuda, Sukrasno, Elfahmi, Totik Sri Mariani. Plant Tissue Culture in Two Varieties of Orthosiphon aristatus (Blume) Miq. JOJ Hortic Arboric. 2019; 2(5): 555597. DOI: 10.19080/JOJHA.2018.01.555597
Abstract
Orthosiphon aristatus has been widely used traditionally for treating several diseases and conditions such as diuretics, rheumatism, abdominal pain, inflammation of the kidneys and bladder, edema, gout, and hypertension. O. aristatus varieties are white flowers, purple flowers, and white-purple flowers. The most populated O. aristatus is purple and white purple shades, but the most commonly used for treatment is purple variety O. aristatus. The content of metabolites the main secondary to O. aristatus is sinensetin, rosmarinic acid, and eupatorin. The level of secondary metabolites in the O. aristatus is still small, so efforts need to be made to increase their level, one of which is plant tissue culture techniques. In this experiment, callus and shoot induction were carried out from two varieties of O. aristatus. Leaf explants of two varieties of O. aristatus were grown on Murashige and Skoog (MS) media with the addition of 3% sucrose and for the induction of callus growth regulators supplemented with 0.4 ppm 2,4-dichlorophenoxyacetic acid (2,4-D, whereas for the induction of shoots the growth regulator added were 2 ppm 6-Benzyl Amino Purine (BAP) and 2 ppm 1-Naphtalen Acetid Acid. Based on the observations, the emergence of callus and shoots occured more quickly in white purple variety than that of purple variety.
Keywords: Plant tissue culture O. aristatus. purple and white-purple varieties Callus and Shoots
Introduction
O. aristatus in Southeast Asia, are widely used to treat rheumatoid disease, diabetes, hypertension, inflammation of the tonsils, epilepsy, impaired menstruation, gonorrhea, syphilis, disorders of the kidney, gall stones, lithiasis, edema, fever, and hepatitis Ameer [1]. The leaves were introduced to Europe and Japan as health tea. O. aristatus are very well known for their diuretic effects, which are stronger than any other natural diuretic. For urinary tract infections treated with fresh leaf decoction is taken twice a day. The dry leaf decoction is often used for the treatment of dysuria. The entire plant is either dried or fresh used to treat kidney stones by traditional medical practitioners Muslihah [2]. People in the Philippines also process decoction of leaves of O. aristatus to get rid of gout De Padua LS [3]. The young twigs and O. aristatus are used to treat back pain in Malaysia Chai [4]. According to Lai Keng & Poay Siong [5] diversity of O. aristatus plants, morphology generally can be seen from the morphology of flowers and leaves. Observation the morphology of O. stamineus shows that purple varieties have a tinge of deafness on the white crown and have colored petals dark red. The leaves are ovoid, green with yellow spots on the upper and lower surfaces of the leaves and the bones of the leaves are purple. The white variety has white flowers and colored petals green. The leaves are rhombic, green and the bones of the leaves light green. O. aristatus are conventionally propagated through cuttings and seeds. The study shows that plant O. aristatus that grow on the ground produce inconsistent bioactive compounds Lee [6]. This issue, along with a variety growth harbor plant in the land, has resulted in plant material O. aristatus that have a non-uniform quality. In vitro, culture techniques such as plant tissue culture can be used as an alternative way to produce plants with uniform quality and quantity of secondary metabolites.
Sterilized Explants
Sterilization of explants two varieties O. aristatus is done by washing the leaves in running water and soaking them in a detergent solution for 5 minutes, then soaking them with dithane-45 0.2% solution for 5 minutes. In the laminar airflow cabinet, sterilization is continued by soaking explants in succession, ie at alcohol 70% for 1 minute, bayclin 20% plus 3 drops of tween 80 for 5 minutes, then rinsed with sterile water three times.
Callus Induction Two Varieties Orthosiphon aristatus (Blume) Miq
Sterile leaf explants two varieties O. aristatus are cut to a size of approximately 1 cm and then planted on the treatment medium MS + Sucrose 3% + 2.4 D 0.4 ppm. Explants that had been plantedin culture bottles were stored in an incubator at 22 oC. Long irradiation with lights 12 hours per day. Observation every day for 3 weeks after planting.
Shoot Induction Two Varieties Orthosiphon aristatus (Blume) Miq
Sterile leaf explants two varieties O. aristatus are cut to a size of approximately 1 cm and then planted on the treatment media MS + Sucrose 3% + BAP 2 ppm + NAA 2 ppm. Explants that had been planted in culture bottles were stored in an incubator at 22 oC. Long irradiation with lights 12 hours per day. Observation every day for 1 month after planting.
Results and Discussion
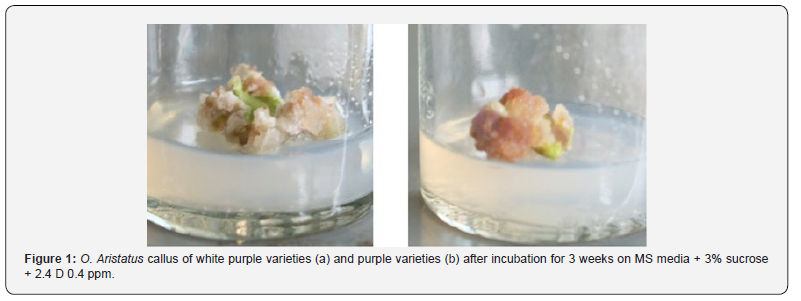
Sterilization of the leaves explants of two varieties of O. aristatus using alcohol 70% for 1 minute and bayclin 20% plus 80 tween for 5 minutes can give good results where the growth media are not easily overgrown with bacteria and fungi for 3 months observation. In the callus induction of two varieties of O. aristatus with MS media + 3% sucrose + 2.4 D 0.4 ppm. After 6 days of observation, the white purple varieties callus were formed, while the purple varieties formed callus on the 11th day and after 3 weeks of observation of the white purple varieties callus showed a larger size than the purple varieties (Figure 1). In shoots induction of two varieties of O. aristatus with MS medium + 3% sucrose + BAP 2 ppm + NAA 2 ppm. After 22 days of observation, new shoots were formed in the white purple varieties, whereas in purple varieties no shoots have formed (Figure 2). Growth regulators are needed as a media component for growth and differentiation. Without the addition of growth regulators in the medium, growth is very inhibited and may not even grow at all. The formation of plant organs is determined by the use of appropriate growth regulators Hendaryanto & Wijayani [7]. This substance is active at low concentrations and toxic at high concentrations. At this time there are six groups of growth regulators known as auxin, gibberellins, cytokinins, abscisic acid (ABA), ethylene, and retardant. Auxin functions for cell division, cell extension, tissue enlargement, and root formation. At high concentrations will stimulate callus growth but inhibit the growth of shoots and roots.

The most effective hormones are 2.4 dichlorophenoxy acetic acid (2,4 D), naphthalene acetic acid (NAA), indole acetic acid (IAA), indole butyric acid (IBA), and pchlorophenoxy acetic acid (pCPA). Cytokines function in shoots induction. Materials that areoften used include kinetin, benzyl adenine (BA) or 6-benzyl amino purine (BAP), zeatin, and isophentenyladenine (2iP). At high concentrations (1 to 10 mg / L) can encourage bud growth, but can inhibit root growth Zulkarnain [8]. The difference in callus and shoot growth time in the two varieties of O. aristatus can be due to genetic differences that exist between the two varieties, as some researchers have previously reported. Research at the genetic level on O. aristatus has been carried out, according to Tnah [9] where microsatellite markers tested can be used to distinguish between white and purple varieties. Other research reported fingerprinting based on molecular data to show that 28 accessions O. aristatus divided by two main groups. Varieties are categorized as white variety O. aristatus, while Kluster II is purple variety O. aristatus. Results of phylogenetic tree analysis showed that two varieties of white and purple O. aristatus are divided into several subspecies Nurul [10]. A species can contain two or more subspecies which usually have some genetic differences compared to each other USDA [11].
Perspective and Conclusion
The addition of growth regulator 2.4 D 0.4 ppm and BAP 2 ppm + NAA 2 ppm can induce callus and shoots of O. aristatus. Callus and shoots are more quickly formed in the white purple variety. These results form the basis of the development of tissue culture from two varieties of a variety of. A callus that is formed into the raw material of cell suspension culture is modified by the addition of precursors and elicitors, so as to increase the levels of secondary metabolites. The shoots formed will then be planted in soil media (acclimatization) to produce seedlings of O. aristatus that have good quality and quantity of secondary metabolites.
References
- Ameer OZ, Salman IM, Asmawi MZ, Ibraheem ZO, Yam MF (2012) Orthosiphon stamineus: Traditional Uses, Phytochemistry, Pharmacology, and Toxicology, Journal of Medicinal Food 15(8): 678-69
- Muslihah F (2007) Tanaman Obat Keluarga (2nd), Penebar Swadaya, Jakarta, Indonesia.
- De Padua LS, Lugod GC, Pancho JV (1987) Handbook on Philippine Medicinal Plants, University of Philippines, Los Banos, USA.
- Chai PPK (2006) Medicinal plants of Sarawak, Malaysia.
- Lai Keng C, Poay Siong L (2006) Morphological Similarities and Differences between the Two Varieties of Cat`s Whiskers (Orthosiphon stamineus Benth.) grown in Malaysia. International Journal of Botany 2(1): 1-6.
- Lee W (2004) Micropropagation and cell culture of misai kucing (Orthosiphon stamineus Benth.) and detection of rosmarinic acid in the in vitro cultures, Tesis, Universiti Sains Malaysia.
- Hendaryanto DPS, Wijayani A (1994) Teknik Kultur Jaringan, Pengenalan dan Petunjuk Perbanyakan Tanaman secara Vegetatif-Moderen, Kanisius, Yogyakarta, Indonesia.
- Zulkarnain Z (2009) Kultur Jaringan Tanaman: Solusi Perbanyakan Tanaman Budidaya, Bumi Aksara, Jakarta, Indonesia.
- Lee Hong, Chai Ting Lee, Song Leong Lee, Chin Hong Ng, Kevin Kit Siong Ng (2014) Development and characterization of microsatellites of an important medicinal plant Orthosiphon stamineus (misai kucing), Biochemical Systematics and Ecology 55: 317-321.
- Nurul, Ain (2015) Study of Molecular and Genetic Diversity of Java Tea (Orthosiphon stamineus Benth.) as A Basis for Plant Improvement. Tesis. Universiti Sains Malaysia.
- USDA, Agricultural Statistics 2006 United States (2006).






























