Growth Stimulation of Phaseolus vulgaris L Plantules by Strain Bacillus amyloliquefaciens Hla Producer of Beneficial Agricultural Enzymes
Slimane Mokrani1,2, Lakhder Belabid2, Billel Bedjaoui2 and Elhafid Nabti1*
1University of Bejaia, Algeria
2Department of Agronomy, Laboratory of Research on Biological Systems, University of Mustapha Stumbouli, Algeria
Submission: July 17, 2018; Published: August 27, 2018
*Corresponding author: Elhafid Nabti, FSNV, Laboratory of Renewable Energies, Group of Biomass and Environment, University of Bejaia, Targa Ouzemmour, Bejaïa, Algeria, Email: elhnabti1977@yahoo.fr University of Agriculture Abeokuta (FUNAAB), Nigeria; Email: otusanyaoluleke@gmail.com
How to cite this article: Slimane M, Lakhder B, Billel B, Elhafid N. Growth Stimulation of Phaseolus vulgaris L Plantules by Strain Bacillus amyloliquefaciens Hla Producer of Beneficial Agricultural Enzymes. JOJ Hortic Arboric. 2018; 2(2): 555581.
Abstract
The strain HLA was identified as Bacillus amyloliquefaciens. Colonies were small and growth rapidly on TSA medium. Microscopic observation with Gram staining and epi-fluorescence revealed Gram positive and long bacilli bacteria. Strain HLA 16S rDNA similarities to the respective species were greater than (79 %). The phylogenetic analysis grouped Strain HLA with strain Bacillus amyloliquefaciens NR 117946.1 (95 %) and two strains of Bacillus subtilis NR113265.1 and NR112116.1 (95%). Strain HLA showed positive results for production of lecithinase, gelatinase and amylase. Inoculation of Phaseolus vulgaris L seed with Bacillus amyloliquefaciens HLA resulted on significative growth stimulation of plantules compared to the control, within 21 days of culture in pots. Bacillus amyloliquefaciens HLA increased, stem length (34.08 %), leaves area (96.5 %), root fresh weight (46.15 %) and root dry weight (70.41 %).
Introduction
In the face of increasing threats to global food security and pressure on natural resources, there is an urgent need to identify and market biologically based products as agricultural crop protectants and yield enhancers [1]. The common bean (Phaseolus vulgaris L.) is the most important pulse crop in the world. It is an important source of calories, proteins, dietary fibers, minerals, and vitamins for millions of people in both developing and developed countries worldwide [2]. Annually, more than 143.88 million nutrient tonnes of chemical fertilizers are used worldwide to increase the yield of crop plants [3]. Despite their efficiency in promoting crop yield, they can, under certain circumstances, pollute the environment and contribute to a number of human and animal health problems [3]. Excessive fertilization is a potential global threat to the soil and environment through soil salinity, acidification as well as neutralization of the soil, heavy metal accumulation, water eutrophication, and accumulation of nitrate [4]. Therefore, studies were aiming to replace chemicals by biofertilizers microorganisms. Biofertilizers (fertilizers of a microbial origin) are eco-friendly and can reduce hazards effects caused by uncontrolled application of chemical inputs [5]. Biofertilizers have been an alternative to mineral fertilizers to increase the yield and plant growth in sustainable agriculture [6]. Plant growth promoting rhizobacteria (PGPR) play a vital role in crop protection, growth promotion and in the improvement of soil health [7]. Ninety-five percent of Gram-positive soil bacilli belong to the genus Bacillus. Members of Bacillus species are able to form endospores and hence survive under adverse conditions [8]. Bacillus amyloliquefaciens were shown to produce volatile compounds that promote plant growth [9]. Production of enzymes that interfere with fungal pathogenesis. Production of chitinases, glucanases, cellulases, lipases and other lytic enzymes may also contribute to effective biocontrol activity [10].
In this study, strain HLA was identified phenotypicaly and cell microscopy. In addition, qualitative production of beneficial agricultural enzymes. In vivo stimulation plantules growth traits of Phaseolus vulgaris L by strain HLA, as shoot and root system, were determined.
Materials and Methods
Origin and characterization of strain HLA
Strain HLA was isolated in 2010 from rhizosphere soil of Phaseolus vulgaris L (common bean) from Tighanif Mascara (35°24’ N 0°19’E). The strain HLA was stored in broth TSB with 25% glycerol, subcultured every 12-18 months.
Phenotypic observation of strain HLA
In order to determine the microscopic aspect of the strain HLA, first subcultured onto TSA agar was carried out, Gram staining was then performed, as well as observation by Fluorescence microscopy (Leica DM 4000B) with camera (DFC310 FX), computer and image processing software. Carried out by spreading a drop of strain HLA suspension in sterile distilled water on a slide, then a microscopic observation under a blue lamp and without coloration were done.
Phylogenic Identification of strain HLA
DNA extraction: Colonies in exponential phase were suspended in 500μl of TE buffer containing (10 mM Tris-HCl at pH= 7.4, 1 mM EDTA at pH=8). Followed by centrifugation at 13.846xg for 3min. The pellet formed was re-suspended in 550μl of TE buffer. 17μl (35μg/ml) of lysozyme were added. The mixture was incubated at 37 ℃ for 30 min. 30μl of SDS (10%) were added. The mix was incubated at 37 ℃ for 30min. 100μl NaCl (5 M) and 80μl CTABS/NaCl (10%/0.7M) were versed. The whole compounds were mixed by inversion then incubated at 65 ℃ for 10min. 800μl of CAI (chloroform/Isoamyl alcohol) were added. The mixture was centrifuged at 13.846 xg for 30min. The supernatant was recuperated and precipitated by addition of 0.6ml of isopropanol and followed by incubation at -20 ℃ for 1-24 h. After centrifugation at 13.846xg for 5min, the pellet was recovered and washed with ethanol (70%), then centrifuged at 13.846xg for 5min. After drying at 37 ℃, total DNA was dissolved in 100μl of TE buffer and treated with ARNase 1μl (20μg/ml) and incubated at 37 °C for 1 h. Finally, genomic DNA recuperated was conserved at -20 ℃.
DNA amplification: PCR amplifications were performed using the following primers: Amorce Formed 16SF (5’- AGA GTT TGA TCC TGG CTC AG -3’) and Amorce reverse 16SR (5’- CTA CGG CTA CCT TGT TAC GA-3’) for 16S rRNA gene. Amplification was carried out using thermalcycler (BOECO TC PRO, Germany) for microtubes containing volume 25μl: 1μl of ADN and 24μl of relational mixture; each microtube contained: (2.5μL tampon, 2.5μL MgCl2 (20 mM), 0.5μL dNTP (25mM), 0.3μL amorce Formed, 0.3μL amorce Reverse, 0.2 μL Taq polymerase, 17.7μL H2O and 1μL DNA. 35 Cycle were applied: preheating at 94°C for 3min denaturation at 94 ℃for 45s, hybridation at 55 ℃ for 1 min, Elongation at 72°C for 2 min, final elongation at 72 ℃ for 7min and final cooling at 14 ℃ for 7min.
DNAr16s sequencing: ADNr 16s sequencing was carried out by the Laboratory of Microorganisms and Actives Biomolecules of the Faculty of Sciences of Tunis (Tunisia) according to the Applied Biosystems protocol on the automatic sequencer (ABI-model 3730xl). ADNr 16s sequences obtained were compared with the nucleotide sequences of international databases using BLAST (Basic Local Alignment Search Tool Program available on line. The alignments of the nucleotide sequences were carried out by the Clustal W algorithm. The phylogenetic tree was obtained by MEGA 7 software and the neighbor-joining algorithm. Bootstrap values were determined from 100 replicates.
Characterization of beneficial agricultural enzymes
Lecithinase: The strain HLA was streaked on egg yolk agar. Followed by incubation at 30 ℃ for 7 days. The appearance of opaque zones around the colonies indicates the presence of lecithinase.
Lipase: To determinate lipase production, the strain HLA was streaked on Tween 80 nutrient agar supplemented with CaCl2. The Petri plate was incubated at 30 °C for 7 days. The appearance of opaque areas around the colonies indicates the presence of lipase activity.
Pectinase: Diluted nutrient agar (¼), which is equivalent to 1000ml/4, added of 0.5 % pectin was used to determine pectinase production by the strain HLA. The bacterium was streak on the medium and incubated at 30 ℃ for 48h. The appearance of a clear halo around growth indicated the presence of a pectinase [11].
Gelatinase: The strain HLA was streaked in an epindorff tube containing the gelatin agar medium with (12%). The control consisted of an epindorff tube containing unincolated gelatin agar. The two tubes were sealed with parafilm, then incubated at 20 ℃ for 15 days. The tubes are set at 4-5 ℃ for 15min. The liquefaction of gelatin was revealed by the inclination of the agar in the test tube and the solidification of the control tube.
Amylase: The strain HLA was streaked on starch agar. Petri plate awa then incubated at 30 ℃ for 2-7 days. After incubation, the Petri plate was flooded with iodine solution. Appearance of clear zones around the bacterial growth, indicated the presence of an amylasic activity
Growth stimulation of Phaseolus vulgaris L plantules
Seed sterilization and soil preparation: Bean seeds of Phaseolus vulgaris L were desinfected by soaking in calcium hypochlorite (2%) for 30min. Then seed were rinsed 3 times with sterile distilled water. Soil was prepared by mixing it with peat (1/3) then sterilized in the pastor oven at 100 ℃ for 3-4 days. The soil is distributed in pots (Superior Ɵ: 7 cm, Inferior Ѳ: 4cm Height: 8.5cm) containing approximately 136g of soil per pot.
Plantules culture and measure of growth traits: Two treatments were prepared (four pots per batch); one traitement was treated with the strain HLA and another control treatment. Three bean seeds were sown per pot. The seeds of the first treatment were soaked in a suspension of strain HLA at a concentration of 108UFC/ml for 30min. The control treatment consisted of seeds soaked in sterile distilled water. The seeds treated, and the controls were coated with starch. The seeds were sown, and the crop was followed for 21 days, under laboratory conditions. The seedlings were watered every two to three days with 10-20ml of sterile distilled water. To evaluate the effects of strain HLA on plantules shoot and root systems growth, traits were measured compared to the control. The measured traits were: stems length, leaves area, fresh and dry weight roots (Burton, 1979). Leaves area were expressed using images of treated and untreated leaves by image J software. Percentages of plantules growth were evaluated using the formula:
PGS = (t – c) /t ×100
Where, PGS: Percentage growth stimulation of each trait, t: trait mean of plantules treated with strain HLA, c: trait mean of control plantules.
Statistic analysis
Growth traits of treated plantules with strain HLA and control were subjected to t- t using statistica.5.0 software at 5%. Traits (SL and DWRB) were analyzed by two-tailed t-test for unequal variances and (LA and WFRB) were analyzed by t-test for equal variances. Normality of the distributions was verified by W test of Shapiro-Wilk and variances homogeneity was verified by Levene test.
Results
Phenotypic observation of strain HLA
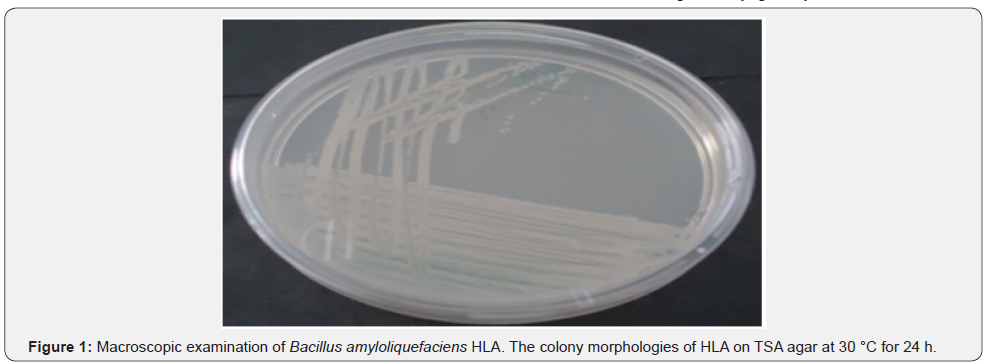
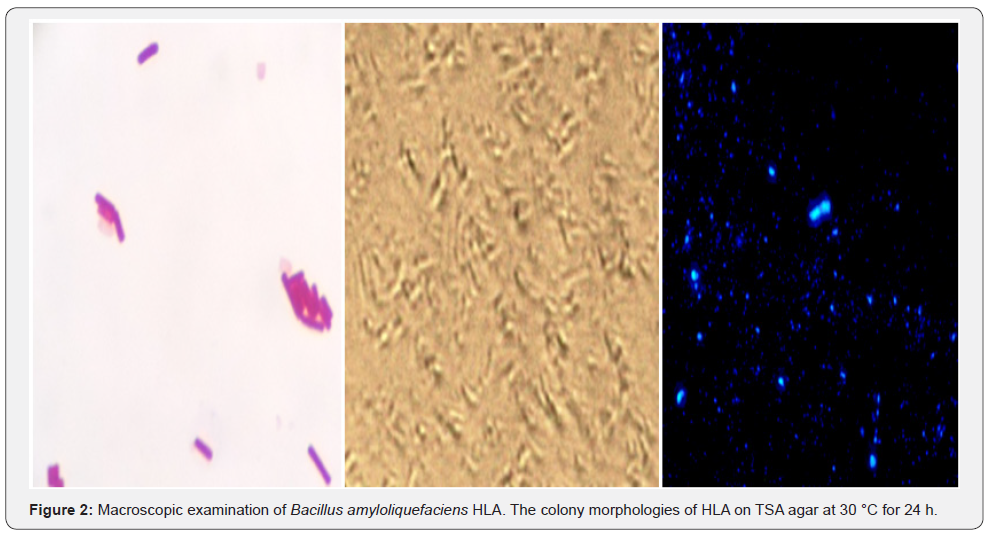
Macroscopic examination of strain HLA revealed that colonies were creamy-white and rough and grew rapidly on TSA medium (Figure1). Gram staining and microscopic examination of strain HLA with and without coloration showed, Gram positive bacteria, cells observed were long Bacilli (Figure 2).
Phylogenic Identification of strain HLA
The constructed phylogenetic tree using the 16s rRNA gene sequences of the related strain HLA from Bacillus amyloliquefaciens and Bacillus subtilis (Figure 3). The phylogenetic tree clearly showed that the strain Bacillus HLA could be divided into two closely related clades, separated by a long Branch length, which indicates an unlimited number of nucleotide changes. Bacillus amyloliquefaciens HLA belong to one clade that was closely related to the B. amyloliquefaciens strain NR 117946.1 (GenBank accession). Four strains made another clade that comprised to references strains Bacillus subtilis NR113265.1 and NR 112116.2. These results were supported by the BLAST search analysis against published 16S rRNA gene sequences in the GenBank database.

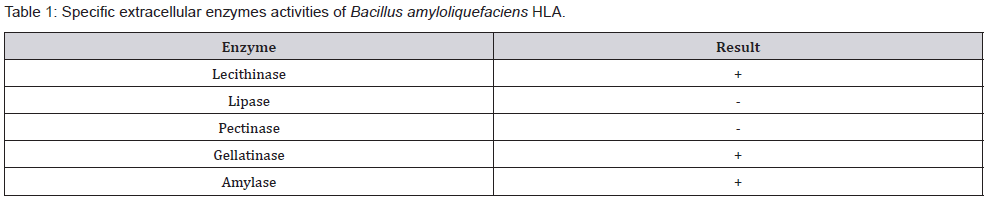
Characterization of beneficial agricultural enzymes: In the present study the extracellular lecithinase, lipase, pectinase, gelatinase and amylase activities of Bacillus amyloliquefaciens HLA were qualitatively evaluated (Table 1). Strain HLA showed positive results for production of lecithinase, gelatinase and amylase. Negative production of lipase and pectinase.
+ and -: positive and negative production.
Growth stimulation of Phaseolus vulgaris L plantules
In vivo study of effect of strain HLA on Phaseollus vulgaris L revealed an increase growth of treated plantules, within 21days (Figure 4). The application of strain HLA revealed showed clearly that treated plantules were high developed, leaves and stems were largest, compared to the control.

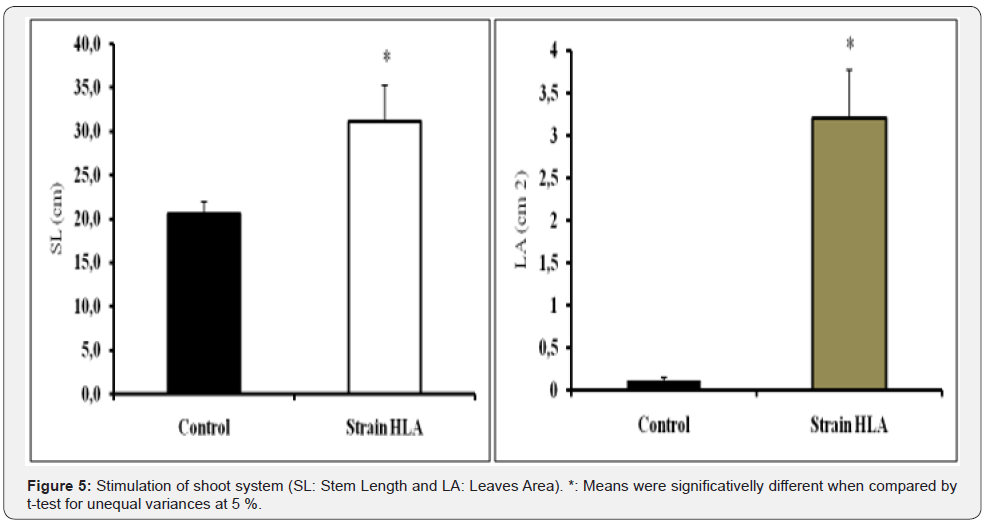
Stimulation of shoot system: Application of strain Bacillus amyloliquefaciens HLA induced growth stimulation of shoots system and stem length (SL) of Phaseolus vulgaris L seedlings (Figure 5). Leaves area (LA) were characterized by significative growth area compared to the control. The leaves area increased to 3.2±0.566cm2 (96.5%) for the plantules treated with strain HLA compared to the control which represented leaves area of 0.112±0.032cm2. Stem length of the treated pantules with strain HLA, increased to 31.1±4.1cm (34.08%) control 20.5±1.4cm.
Stimulation of root growth: Strain Bacillus amyloliquefaciens HLA induced also growth of plantules root system (Figure 6). The root systems were visually affected, revealing clearly that root treated with a strain HLA were high compacted and condensated compared to the control. The dry and fresh weight roots of plantules treated by strain Bacillus amyloliquefaciens HLA increased significative (Figure 7). The fresh weight of the root biomass (WFRB) increase to 1.3±0.3g (46.15%) compared to the control of 0.7±0.4g. Also, the dry weight of the root biomass (DWRB) increased to 0.1893±0.0445g (70.41%) compared to the control 0.0560±0.0158g [12].
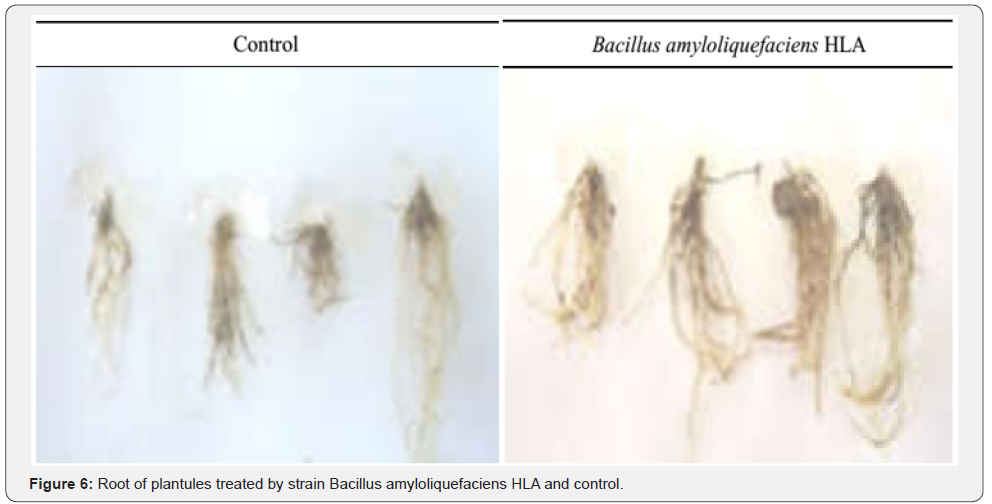
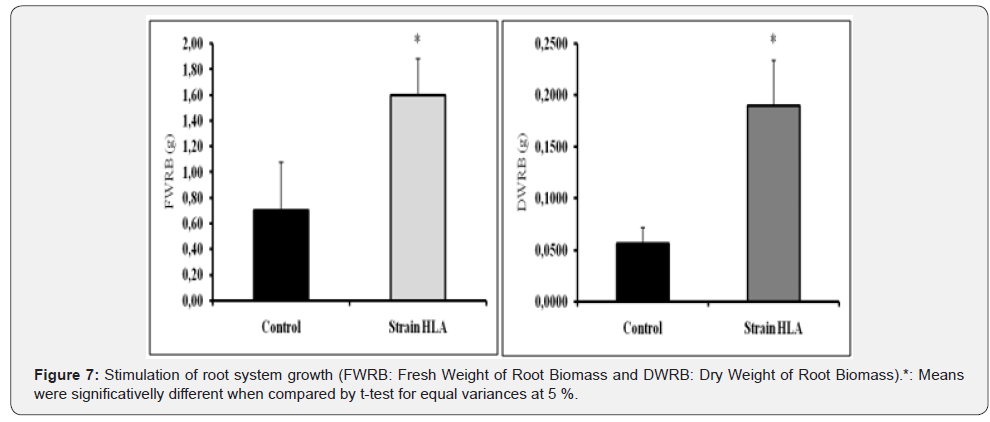
Discussion
The use of PGPR to enhance plat crops is an environment friendly approach and an effective alternative to toxic chemical fertilizers. Microorganisms that presents several biologic activities of agricultural interest, practically, can make them subject of biofertilizing object, for this the enzymatic tests play a crucial role in the microbial screening [13]. Those bacteria produce extracellular enzymes as strategies to join skills to get ecological advantages [13]. Pectinase is a group of enzymes that known to catalyse the pectic substance through depolymerisation and deexrification reaction. These enzymes have the role in preventing plant from infection caused by pathogen [11]. Protease, which can degrade cell wall proteins; and lipase, which can degrade some of the cell wall-associated lipid, all of which can to some extent individually lyse fungal cells [14]. Bacillus spp. strains are an effectiveness soil bacterium characterized by various enzymatic activity with implications either in cell wall degradation of plant pathogenic microorganisms, or in metabolism of various substrates. Those enzymes included cellulases, chitinases, proteases (gelatinases) and lipases [15]. Production of chitinases, glucanases, cellulases, lipases and other lytic enzymes may also contribute to effective biocontrol activity [16].
In this study, inoculation of Phaseolus vulgaris L seed with strain Bacillus amyloliquefaciens HLA increased plants growth. Growth traits of shoot and root system were signivicativelly increased there is no single mechanism for promoting plant growth [17]. The mechanisms by which bacteria can influence plant growth differ among species and strains, so typically PGPR exsers their effects on root tissues, can modify the physiology and functioning of plant tissues located at a substantial distance from the colonized sites, such as shoots. Two types of mechanisms are involved. On the one hand, some PGPR can enhance nutrient availability/uptake for plant roots. On the other hand, certain PGPR trigger specific systemic responses, mostly by unknown signaling mechanisms [18].
Sufficient densities of PGPR in biofertilizer provide a beneficial role in creating a proper rhizosphere for plant growth and converting nutritionally important elements through biological process, for example increasing the availability of N, P, K, as well as inhibiting pathogen growth [19]. The high availability of N, P, and K could enhance soil fertility, improve antagonistic isolates’ bio-control effects, and extend microorganisms’ survival rates in soil [20]. By far the most evidence for the positive effects of biofertilizing-PGPR points to bacteria-mediated changes in root growth and morphology. Bacterial mediated increases in root weight are commonly reported responses to GPR inoculations.
Sabaté et al. [21] reported that B. amyloliquefaciens in greenhouse experiments showed that the black common bean cv. Nag 12 seeds inoculated with B14, had increased germination of 10%, as well as an increase in root length of 2cm and in shoot length of 6 cm compared with the non-inoculated control seeds. PGPR treatments increased fresh and dry shoot, stem diameter, seedling height, chlorophyll reading values, and leaf area of cabbage seedlings. Such an improvement might be attributed to the N2-fixing and phosphate-solubilizing capacities of bacteria, as well as the ability of these microorganisms to produce growthpromoting substances [22].
Conclusion
Strain Bacillus amyloliquefaciens HLA, identified and used in this present study as a biofertilizer of growth of Phaseolus vulgaris L shows that it is a promising natural antagonist. It exhibited sufficient enzymes with beneficial agriculture application, that can play as the same time role on suppressing pathogens and stimulation plant growth by degradation of macromolecules/ nutriments in soil.
Acknowledgment
Research funding from Laboratory of Biotechnology and Bio- Geo Resources Valorization, Higher Institute for Biotechnology, University of Manouba, Ariana, (Tunisia) and Laboratory of Microorganisms and Actives Biomolecules of the Faculty of Sciences, Tunis (Tunisia).
References
- Velivelli SL, De Vos P, Kromann P, Declerck S, Prestwich BD (2014) Biological control agents: from field to market, problems, and challenges. Trends Biotechnol 32(10): 493-496.
- Singh SP (2013) Common bean improvement in the twenty-first century. Springer Science & Business Media, pp. 7
- Naveed M, Zahir ZA, Khalid M, Asghar HN, Akhtar MJ, et al. (2008) Rhizobacteria containing ACC-deaminase for improving growth and yield of wheat under fertilized conditions. Pakistan Journal of Botany 40: 1231-1234.
- Savci S (2012) An agricultural pollutant: chemical fertilizer. International Journal of Environmental Science and Development 3: 77-80.
- Hegazi AZ, Khattab EA, Shehata HS, Mostafa SSM (2015) Application Efficiency of Spent Mushroom Compost Extract, Cyanobacteria and Bacteria on Green Fruit and Seed Yield of Squash under Drip Irrigation System. Middle East J Agric Re 4: 887-898.
- Canbolat MY, Barik K, Çakmakçi R, şahin F (2006) Effects of mineral and biofertilizers on barley growth on compacted soil. Acta Agriculturae Scandinavica Section B-Soil and Plant Science 56(4): 324-332.
- Fernando W D, Nakkeeran S, Zhang, Y (2005) Biosynthesis of antibiotics by PGPR and its relation in biocontrol of plant diseases. In PGPR: Biocontrol and Biofertilization. Springer Netherlands 3: 67-109.
- Ahmad I, Pichtel J, Haya S (2008) Plant-bacteria Interactions: Strategies and Techniques to promote plant growth. John Wiley & Sons, USA.
- Ryu CM, Farag MA, Hu CH, Reddy MS, Wei HX et al. (2003) Bacterial volatiles promote growth in Arabidopsis. Proceedings of the National Academy of Sciences 100(8), 4927-4932
- Beattie GA (2007) Plant-associated bacteria: survey, molecular phylogeny, genomics and recent advances. In Plant-associated bacteria Springer, Dordrecht 1: 1-56.
- Reetha S, Selvakumar G, Bhuvaneswari G, Thamizhiniyan P, Ravimycin T (2014) Screening of cellulase and pectinase by using Pseudomonas fluorescens and Bacillus subtilis. International Letters of Natural Sciences 8 :75-80.
- Sicard R, Reymond JL (2006) Introduction: Enzyme assays: Highthroughput screening, genetic selection and fingerprinting. In: Reymond, JL (Ed), Wiley VCH Verlag Gmbh & Co. KGaA, Weinheim, Germany: 7-9.
- Fogliano V, Ballio A, Gallo M, Woo S, Scala F, et al. (2002) Pseudomonas lipodepsipeptides and fungal cell wall-degrading enzymes act synergistically in biological control. Molecular plant-microbe interactions 15(4): 323-333.
- Friedrich N, Hagedorn M, Soldati FD, Soldati T (2012) Prison break: pathogens’ strategies to egress from host cells. Microbiology and Molecular Biology Reviews 76(4): 707-720.
- Sicuia OA, Grosu I, Constantinescu F, Voaideş C, Cornea CP (2015) Enzymatic and genetic variability in Bacillus spp. strains with plant beneficial qualities. AgroLife Scientific Journal 4(2): 124-131.
- Souza RD, Ambrosini A, Passaglia L M (2015) Plant growth-promoting bacteria as inoculants in agricultural soils. Genet Mol Biol 38(4): 401- 419.
- Vacheron J, Desbrosses G, Bouffaud ML, Touraine B, Moënne-Loccoz Y, et al. (2013) Plant growth-promoting rhizobacteria and root system functioning. Front Plant Sci 17(4) :356.
- Waddington SR (1998) Organic matter management: From science to practice. Soil Fertil 62: 24-25.
- Yang X, Chen L, Yong X, Shen Q (2011) Formulations can affect rhizosphere colonization and biocontrol efficiency of Trichoderma harzianum SQR-T037 against Fusarium wilt of cucumbers. Biology and Fertility of Soils 47(3): 239-248.
- Vessey JK (2003) Plant growth promoting rhizobacteria as biofertilizers. Plant and soil 255(2): 571-586
- Sabaté DC, Brandan CP, Petroselli G, Erra-Balsells R, Audisio MC (2017) Decrease in the incidence of charcoal root rot in common bean (Phaseolus vulgaris L) by Bacillus amyloliquefaciens B14, a strain with PGPR properties. Biological Control 113: 1-8.
- Turan M, Ekinci M, Yildirim E, Güneş A, Karagöz K (2014) Plant growthpromoting rhizobacteria improved growth, nutrient, and hormone content of cabbage (Brassica oleracea) seedlings. Turkish Journal of Agriculture and Forestry 38: 327-333.






























