Abstract
Background information: Onychomycosis is a prevalent and persistent fungal infection of the nails, primarily caused by Trichophyton rubrum, posing therapeutic challenges due to poor drug penetration and high relapse rates. While oral antifungals like terbinafine remain effective, topical alternatives and physical adjuncts such as laser therapy are being explored to enhance efficacy and safety.
Objective: This randomized prospective assessor-blinded clinical trial aimed to evaluate the efficacy of combining 980 nm diode laser therapy with a terbinafine nail lacquier formulated in a hydro-alcoholic solution of hydroxy-propyl chitosan (Onicoter, Cantabria Labs Difa Cooper) compared to laser therapy alone in the treatment of mild-to-moderate onychomycosis.
Methods: A total of 111 patients were randomized in a 1:2 ratio to receive either laser therapy alone (Group A, n=37) or laser therapy followed by topical terbinafine nail lacquer application (Group B, n=74). Laser treatment was administered weekly for three sessions, followed in Group B by daily lacquer application for four weeks and weekly thereafter for six months. Clinical parameters—nail involvement area, dyschromia, and dystrophy—were assessed at baseline (V0), post-laser (V2), six months (V3), and twelve months (V4). Statistical significance was determined using Welch’s t-test (p < 0.05).
Results: Both groups exhibited progressive clinical improvement. While reduction in nail involvement area was observed in both arms, no statistically significant differences were found between groups. However, at V4, Group B demonstrated significantly greater improvements in nail dystrophy and dyschromia compared to Group A (p < 0.05), supporting the added benefit of the lacquer.
Conclusion: The sequential combination of diode laser therapy and terbinafine nail lacquier formulated in a hydro-alcoholic solution of hydroxy-propyl chitosan is more effective than laser therapy alone in improving nail dyschromia and dystrophy in onychomycosis. This dual approach offers a promising, well-tolerated alternative to systemic antifungal regimens, potentially improving patient outcomes and adherence.
Keywords:Dermatophyte nail infections; Lacquer Terbinafine; Scopulariopsis; Chronic onycholysis
Introduction
Androgenetic alopecia (AGA) is a common hereditary condition characterized by progressive hair thinning in genetically predisposed individuals [1]. It represents the most prevalent form of hair loss, particularly among Caucasian men, with epidemiological studies estimating that up to 80% of males will experience some degree of AGA by the age of 70 [2]. AGA presents differently between sexes. Men typically experience bitemporal, frontal and vertex thinning, or complete hair loss while women show diffuse thinning over the crown, usually sparing the frontal hairline [3]. AGA is an androgen-dependent condition, with both hormone metabolism and androgen receptor activity playing central roles in its pathogenesis. Affected individuals show increased levels of dihydrotestosterone (DHT), elevated activity of 5-alpha-reductase, and a higher density of androgen receptors in balding scalp regions. DHT binding promotes follicular miniaturization by altering gene expression. This disrupts the normal hair cycle by shortening the growth (anagen) phase, leading to progressively finer and shorter hairs.
Several treatments aim to slow its progression and boost hair regrowth. These include oral, topical, physical, and injectable approaches. Among injectable treatments, platelet-rich plasma (PRP) and polynucleotides (PN) have gained attention for their regenerative potential. PRP involves injecting autologous platelet-rich plasma into the scalp and is typically recommended in the early-stages, when hair follicles remain intact [4-6]. PRP activates platelets, leading to the release of a variety of growth factors that promote follicular regeneration and hair growth [7]. Additionally, PRP may counteract DHT process through elevated IGF-1 levels, supporting follicular survival and activity. This treatment is generally well tolerated but potential side effects include mild scalp irritation, sensitivity, and scaling; rarely, injection-site infections may occur.
PRP is contraindicated in cases of malignancy, blood disorders, anaemia, immunosuppression, or pregnancy [4]. On the other side, PN HPTTM are DNA-derived macromolecules of varying lengths, naturally sourced from the gonads of freshwater-bred trout, Oncorhynchus mykiss, raised for human consumption. PN HPTTM supports hair growth by fostering a favorable follicular microenvironment, enhancing the trophism of hair bulb cells and enhancing cell proliferation, tissue elasticity, and scalp hydration. These combined effects contribute to stronger, healthier, and more resilient hair [8-10]. Several extraction processes have been developed to produce sterile, injectable-grade polynucleotides.
Among these, Mastelli S.r.l. (Sanremo, Italy) has introduced High Purification Technology (HPT™), a proprietary method for the extraction and purification of PN HPT™ from trout gonads [8-11]. This process yields PN HPT™, a highly biocompatible, naturally derived functional ingredient, free from protein contaminants and devoid of pharmacological or allergenic potential. PN HPT™ has demonstrated a reconditioning effect on the dermal environment and has been associated with improved follicular health. PN HPT™ exert their activity by adequately stimulating fibroblasts, whose paracrine signals are essential for initiating and sustaining follicular regeneration and hair follicle neogenesis [8-10].
These PN HPT™ are available in a variety of formulations designed to meet diverse clinical needs. Despite its high prevalence, AGA remains a therapeutic challenge due to variability in clinical presentation and response to treatment. While not life-threatening, the psychosocial burden of hair loss can significantly impact quality of life, underscoring the importance of developing and evaluating novel therapeutic strategies [12]. This case series reports the single-centre experience of Plinest Hair in 11 patients with AGA. Each patient received Plinest Hair on one side of the scalp, and PRP on the contralateral side.
Case Report
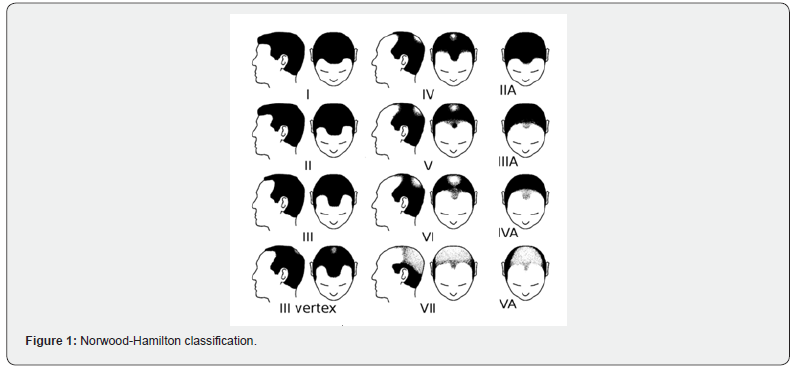
Data from 11 male patients diagnosed with androgenetic alopecia between 18 and 50 years old were collected and analysed to confirm the safety and efficacy of Plinest Hair (Mastelli S.r.l.). After providing the subjects with full information and receiving their consent, half of the patients’ scalp were treated with Plinest Hair, while the other half received a PRP injection. The data collected for each patient included demographic and historical information. The hair loss pattern was graded using the widely accepted Hamilton-Norwood scale (HNS), which consists of seven stages that define the pattern and progression of hair loss (Figure 1) [13].
The efficacy of the treatments was evaluated focusing on the following parameters:
• Hair growth and density using a high magnification micro camera with a 1 cm² snapshot and qualitative assessment.
• Hair Loss Count (Combing Test): Patients comb their hair starting from the crown toward the forehead, sides, and back, allowing fallen hairs to collect on a white towel. After 60 seconds of combing, the total number of fallen hairs is counted.
• Clinical improvement and satisfaction using the Global Aesthetic Improvement Scale (GAIS) by both the patient and investigator. GAIS is a subjective, qualitative tool used primarily in aesthetic medicine to assess changes in a patient's appearance. Both patients and investigators use GAIS to rate overall improvement from baseline, typically categorizing results into descriptors. The rating range between 1 “very much improved” and 5 “worse”.
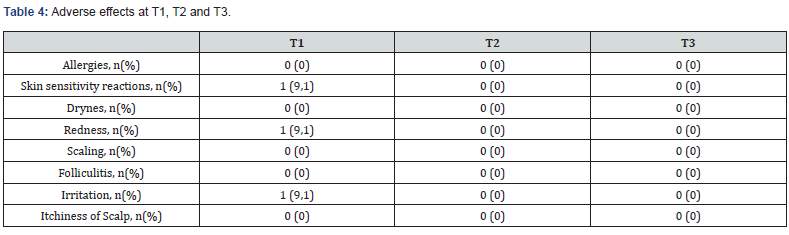
Safety of the Plinest Hair treatment was evaluated by monitoring local adverse events, including dryness, scaling, redness, folliculitis, irritation and itchiness. Additionally, injection-site pain was assessed both for Plinest Hair and PRP treatments, using the Numeric Pain Rating Scale (NPRS) from 0 (no pain) to 10 (worst possible pain) [14]. Each patient provided a pain rating after each injection on both sides of the scalp. Mean pain scores were calculated per injection and per patient. Furthermore, data concerning the usability of Plinest Hair was collected through a qualitative scale, evaluating handling, injectability, and extrudability of the device.
The Plinest Hair treatment protocol consisted of eight intradermal injection sessions targeting the superficial dermis of the scalp over a three-month period:
• Month 1: Four injections, administered at weekly intervals.
• Months 2 and 3: One injection every two weeks (four injections in total).
Plinest Hair is a sterile, single-use, viscoelastic polynucleotide gel for intradermal administration. Each pre-filled syringe containing 15 mg of PN HPTTM derived from trout, suspended in 2 ml of fluid gel. During each session, 2 ml were injected intradermally using a 30G needle in 0.1 ml aliquots spaced 1-2 cm apart across the affected areas to promote cell growth, metabolism, and regeneration.
Platelet-Rich Plasma (PRP) therapy is a regenerative approach increasingly employed in the management of androgenetic alopecia. The standard preparation and application involve the following steps:
• Blood Collection: Approximately 10 to 60 mL of autologous venous blood is drawn from the patient.
• Centrifugation: The sample is processed in a centrifuge to separate its components by density. This isolates the platelet-rich plasma (PRP) from red blood cells and platelet-poor plasma.
• PRP Extraction: The platelet-rich layer (typically located just above the buffy coat) is collected. This portion contains a concentrated level of platelets suspended in plasma [15-17].
PRP injection protocol consists of four injections sessions over three months:
• Month 1: two injections, one every 2 weeks.
• Months 2 and 3: One injection every 4 weeks (two injections in total).
To match the injection frequency of the Plinest Hair protocol and maintain patient blinding, PRP was administered in alternating sessions with saline placebo injections. Specifically, patients received one PRP session followed by one saline injection, and so on, for a total of eight sessions. This design ensured that both treatment groups underwent the same number of visits and scalp injections, while preserving the double-blind nature of the study. Data collection was conducted at baseline (T0), at the end of the treatment (T1), and at 3 (T2) and 6 (T3) months after the end of treatment.
Patients’ demographic characteristics are outlined in Table 1. The mean age of the subjects was approximately 35 years, and most of them exhibited HNS type III and type III vertex pattern hair loss. The subjects had not previously suffered from any diseases of a trichological nature that required attention or treatment. Most of the population exhibited signs of alopecia for a period ranging from one to five years at the time of the treatment. None of them experienced patchy hair loss.
At the baseline (T0) stage, patients were subjected to a comprehensive evaluation of their hair count and loss, which included both the Global Photographic Assessment and the Combing Test, as outlined in Table 2. The hair loss count for the eight patients exceeded 40 hairs. Most patients exhibited hair thinning in the frontal and parietal regions or in the frontal and temporal regions. Five of the subjects exhibited a vertex pattern of baldness. Two patients were diagnosed with dandruff and seborrheic dermatitis. However, these conditions were not present in either patient at T1.
Results
At the time of analysis at T1, all patients showed signs of improvement. Most cases exhibited a significant enhancement in hair quality and thickness. No noticeable difference in outcomes was observed between the two treatment modalities. At T2, no significant changes were noted. At T3, data was unavailable for seven patients. Among the four remaining patients, two showed sustained results from T1. Table 3 presents the progression of patients through the various treatment phases.
No hair loss was observed at T1 or T2. At T3, only two of the four patients for whom data was available reported experiencing slight hair loss. Treatment efficacy was also assessed using the Global Aesthetic Improvement Scale (GAIS) by both participant and investigator. The results indicated that there were no differences observed between the two treatment groups. Overall, the patient's perspective is marginally inferior to that of the investigator. Participant scores generally ranged between "much improved" and "improved." Local adverse events were documented at T1, T2 and T3 and are illustrated in Table 4. In summary, only three cases had an adverse effect. Specifically, the reactions observed at T1 visit were all mild and resolved spontaneously, these included skin sensitivity, redness, and irritation.
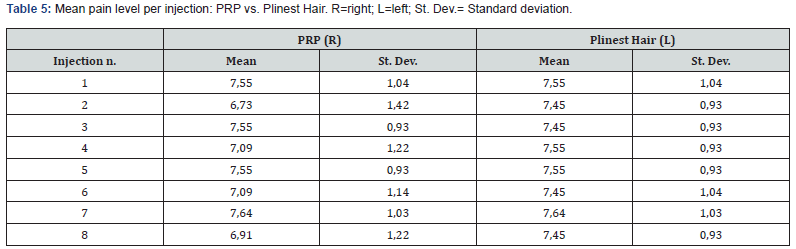
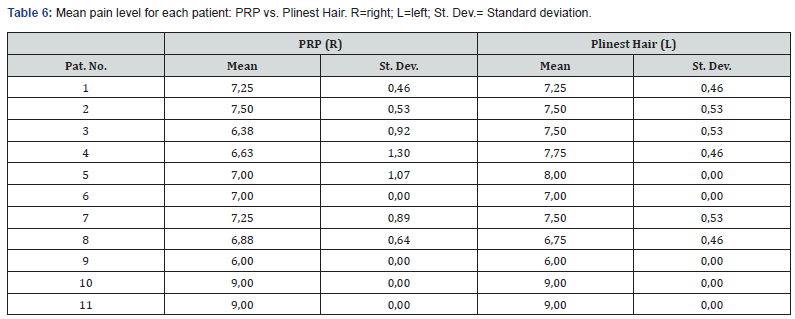
The two treatments were compared in terms of pain perception at each injection. Table 5 shows the mean perceived pain score associated with each of the 8 injections administered. There is no notable difference between the right and the left side of the scalp, i.e. between the two treatments. The analysis of pain perception for each patient assessed subjective pain perception at the individual level (Table 6). Throughout the injections, the pain did not vary greatly. Pain was reported ranging from 6 to 9 for both for the PRP and the Plinest Hair treatments.
Investigators also assessed the usability of the Plinest Hair Intradermal Polynucleotide Gel (Mastelli S.r.l.) device, evaluating factors such as handling, injectability and extrudability in a clinical setting. At the end of the treatment period, each parameter was qualitatively assessed for every patient, resulting in a total of 11 evaluations per parameter. In 54% of cases, the device's handling and extrudability were rated as "good," while its injectability was considered "good" in 90% of cases.
Discussion
The present case series describes a single-centre experience involving 11 male patients diagnosed with androgenetic alopecia (AGA), treated with Plinest Hair (Mastelli S.r.l.) and platelet-rich plasma (PRP). PN HPT™ beneficial effects are largely attributed to their action providing moisturizing and free radical–scavenging effects on dermal fibroblasts [10,18]. Fibroblasts play a central role in the dynamics of hair follicle regeneration, as they release paracrine factors that trigger the formation and regrowth of hair follicles. When fibroblasts become functionally impaired, the health and physiology of the scalp deteriorate.
PN HPT™ help restore an optimal microenvironment for follicular regeneration by stimulating fibroblast activity. Due to this reason, Plinest Hair, an intradermal PN HPT™-based gel derived from gonad trout, represents a valuable therapeutic tool in promoting scalp health and hair restoration. The results evidenced its effectiveness in the management of androgenetic alopecia (AGA), strengthening the hair shaft and counteracting hormonally driven hair loss. The gel formulation, administered via fine-needle injection into the scalp, provides visco-supplementation, offering intradermal support, improved tissue tone, deep hydration, and antioxidant protection [9,10,18-20].
In our series, the use of Plinest Hair in cases of mild hair loss yielded positive outcomes, preventing further hair loss, reducing hair shedding, increasing hair thickness, and improving scalp coverage. Only three mild complications were reported and resolved spontaneously before T2, further confirming the device safety. Indeed, Plinest Hair has demonstrated its safety and efficacy, as previously observed in various applications in aesthetic and plastic medicine, including the correction of moderate-to-severe striae albae [21], treatment of postsurgical atrophic scars [22], correction of nasolabial folds [23], skin regeneration and rejuvenation [24], and acne scar treatment [25]. Across these studies, only a few mild complications emerged, reinforcing the safety profile of PN HPT™ in monotherapy.
Although our experience with Plinest Hair is based on a small cohort, its performance was comparable to that of PRP. Given the substantial evidence supporting PRP’s efficacy in increasing hair density and thickness in patients with AGA [26], the positive outcomes observed with Plinest Hair appear promising. However, while PRP is widely regarded as effective, its results often vary due to inconsistencies in preparation protocols, platelet concentration, and administration techniques [26]. Furthermore, its autologous nature complicates clinical evaluation, as individual factors, such as immune response, medical history, and demographics, can influence treatment outcomes [27,28].
The lack of standardization in PRP preparation affects safety assessments. The multiple steps involved (blood collection, processing, and reinjection) introduce a persistent risk of microbial contamination, with complications including local infections, inflammation, allergic reactions, nodule formation, and in rare cases, blindness [29,30]. A case report published in 2023 documented a 70-year-old female patient who developed herpes zoster ophthalmicus (HZO) with superimposed bacterial infection following PRP treatment for androgenetic alopecia. The authors hypothesized that HZO was caused by acute stress, which was likely induced by PRP treatment [29,30]. In contrast, a product with a standardized formulation, such as Plinest Hair, may offer more consistent and reproducible outcomes, along with a less invasive procedure, as it eliminates the need for blood sampling, thereby reducing patient risk and overall costs [31]. Although PRP typically requires fewer sessions, its high financial burden and issues related to accessibility may limit its use and potentially outweigh its clinical benefits. Given the chronic nature of AGA and its considerable psychological impact on patients, evaluating the cost-effectiveness of these therapies is imperative [27,32]. Injectable treatments represent a safer alternative within the wide array of available options, offering effective means to slow disease progression and stimulate hair regrowth. Oral finasteride, approved by both the FDA and EMA, demonstrated to be effective but as with all systemic therapies may carry a higher risk of side effects compared to topical options [12]. Topical minoxidil, instead, has been demonstrated to enhance scalp vascularization and improve nutrient and oxygen supply to hair follicles [4]. For this reason, is often used as a first-line or adjunctive therapy; nonetheless, side effects such as contact dermatitis, itching, scalp irritation, or facial hypertrichosis have been reported, although discomfort is frequently linked to the application itself rather than to true adverse reactions [12]. Physical treatments, such as low-level laser therapy (LLLT) and hair transplantation have also been mentioned. LLLT may promote angiogenesis and activate follicular stem cells, but minimal side effects have been reported, including isolated cases of acne, mild paranesthesia, dry skin, headache, and pruritus.
The surgical approach, on the other hand, involves relocating androgen-insensitive follicles to balding areas, however it is associated with side effects, including adverse reactions to anaesthesia, bleeding, pain, edema, intraoperative or postoperative pain, and patient dissatisfaction [33]. Our case series confirmed patient satisfaction with this procedure, which ranged between “improved” and “much improved”, without differences between the right (PRP) and left (Plinest Hair) side of the scalp. This result was in line with the investigator's assessment. Within the efficacy evaluation, it is noteworthy that all patients showed improvement at T1, after which their condition remained stable without a decline in effect. Pain levels demonstrated stability over time, whilst inter-individual differences were observed. This variation may be attributed to the subjective nature of pain perception and the procedural aspects, as observed in a recent publication by [34] where patients treated with PRP experienced a huge variability in pain score [34]. This is the first evidence comparing the use of polynucleotides and PRP for AGA. Preliminary findings suggest that Plinest Hair may be a promising treatment option, with comparable efficacy of PRP, though further studies with larger cohorts and extended follow-up are needed to confirm these results. Although the analyses were limited by the small sample size and incomplete follow-up in some patients, PN HPTTM represents a valid standardized treatment option in treating the AGA in a safe and effective way.
- Price VH (1999) Treatment of Hair Loss. N Engl J Med 341(13): 964-973.
- Lolli F, Pallotti F, Rossi A, Fortuna MC, Caro G, et al. (2017) Androgenetic alopecia: a review. Endocrine 57(1): 9–17.
- Phillips TG, Slomiany WP, Allison R (2017) Hair Loss: Common Causes and Treatment. Am Fam Physician 96(6): 371-378.
- Kaiser M, Abdin R, Gaumond SI, Issa NT, Jimenez JJ (2023) Treatment of Androgenetic Alopecia: Current Guidance and Unmet Needs. Clin Cosmet Investig Dermatol 16: 1387-1406.
- Meyers A, Jin A, Kwiecien GJ, Gatherwright J, Khetarpal S, Zins JE (2023) Platelet-Rich Plasma for Treatment of Hair Loss Improves Patient-Reported Quality of Life. Aesthetic Plast Surg 47(4): 1528-1534.
- Singhal P, Agarwal S, Dhot PS, Sayal SK (2015) Efficacy of platelet-rich plasma in treatment of androgenic alopecia. Asian J Transfus Sci 9(2): 159-162.
- Stevens J, Khetarpal S (2019) Platelet-rich plasma for androgenetic alopecia: A review of the literature and proposed treatment protocol. Int J Womens Dermatol 5(1): 46-51.
- Cavallini M, Bartoletti E, Maioli L, Palmieri IP, Papagni M, et al. (2024) Value and Benefits of the Polynucleotides HPTTM Dermal Priming Paradigm A Consensus on Practice Guidance for Aesthetic Medicine Practitioners and Future Research. Clin Exp Dermatol Ther 9: 224.
- Cavallini M, Papagni M (2021) Polynucleotides Highly Purified Technology at the hair follicle level. Aesthetic Medicine J Cosmet Dermatol 7(2).
- Cavallini M, Bartoletti E, Maioli L, Massirone A, Pia Palmieri I, et al. (2021) Consensus report on the use of PN‐HPTTM (polynucleotides highly purified technology) in aesthetic medicine. J Cosmet Dermatol 20(3): 922-92
- Colangelo MT, Govoni P, Belletti S, Squadrito S, Guizzardi S, et al. (2021) Polynucleotide biogel enhances tissue repair, matrix deposition and organization. Journal of biological regulators & homeostatic agents 35(1): 355-362.
- Nestor MS, Ablon G, Gade A, Han H, Fischer DL (2021) Treatment options for androgenetic alopecia: Efficacy, side effects, compliance, financial considerations, and ethics. J Cosmet Dermatol 20(12): 3759-3781.
- Wirya C, Wu W, Wu K (2017) Classification of male-pattern hair loss. Int J Trichology 9(3): 95-100.
- Liu J, Chen J, Tian L, Tang C, Shuai W, et al. (2024) Translation, cultural adaptation, and validation of Numerical Pain Rating Scale and Global Rating of Change in Tibetan musculoskeletal trauma patients. Sci Rep 14(1): 11961.
- Alves R, Grimalt R (2016) Randomized Placebo-Controlled, Double-Blind, Half-Head Study to Assess the Efficacy of Platelet-Rich Plasma on the Treatment of Androgenetic Alopecia. Dermatol Surg 42(4): 491-497.
- Gentile P, Garcovich S, Bielli A, Scioli MG, Orlandi A, et al. (2015) The Effect of Platelet-Rich Plasma in Hair Regrowth: A Randomized Placebo-Controlled Trial. Stem Cells Transl Med 4(11): 1317–1323.
- Gupta AK, Carviel JL (2017) Meta-analysis of efficacy of platelet-rich plasma therapy for androgenetic alopecia. J Dermatol Treat 28(1): 55-58.
- Colangelo MT, Belletti S, Guizzardi S, Galli C (2025) Polynucleotides HPTTM -based compounds exhibit Scavenging Activity Against Reactive Oxygen Species. Preprints
- Samadi A, Ayatollahi A, Kashani MN, Zamani S, Firooz A (2024) Efficacy and tolerability assessment of a polynucleotide-based gel for improvement of pattern hair loss. Arch Dermatol Res 316(6): 331.
- Cavallini M, Bartoletti E, Maioli L, Palmieri IP, Papagni M (2024) Value and Benefits of the Polynucleotides HPTTM Dermal Priming Paradigm A Consensus on Practice Guidance for Aesthetic Medicine Practitioners and Future Research. Clin Exp Dermatol Ther 9: 224.
- Bartoletti E, De Luca C, Maioli L, Moro L, Palmieri IP, et al. (2023) PN HPTTM and Striae Albae-Exploratory Interim Analysis of a Randomised Prospective Study. Surg Res 5(3).
- Araco A, Araco F, Raichi M (2025) An Exploratory Study of PN HPT for Treating Postsurgical Atrophic and Depressed Scars. J Cosmet Dermatol 24(1): e16764.
- Araco A, Araco F, Raichi M (2023) Clinical efficacy and safety of polynucleotides highly purified technology (PN‐HPT®) and cross‐linked hyaluronic acid for moderate to severe nasolabial folds: A prospective, randomized, exploratory study. J Cosmet Dermatol 22(1): 146-1
- Lim TS, Liew S, Tee XJ, Chong I, Lo FJ, et al. (2024) Polynucleotides HPT for Asian Skin Regeneration and Rejuvenation. Clin Cosmet Investig Dermatol 17: 417-431.
- Araco A, Araco F (2021) Preliminary Prospective and Randomized Study of Highly Purified Polynucleotide vs Placebo in Treatment of Moderate to Severe Acne Scars. Aesthet Surg J 41(7): NP866-NP874.
- Lopes-Silva R, Santos M, Sequeira ML, Silva A, Antunes T, Valejo-Coelho P, et al. (2025) Platelet-Rich Plasma Effectiveness in Treating Androgenetic Alopecia: A Comprehensive Evaluation. Cureus 17(1): e77371.
- Gupta S, Paliczak A, Delgado D (2021) Evidence-based indications of platelet-rich plasma therapy. Expert Rev Hematol 14(1): 97-108.
- Cruciani M, Masiello F, Pati I, Marano G, Pupella S, De Angelis V (2021) Platelet-rich plasma for the treatment of alopecia: a systematic review and meta-analysis: PRP for treatment of alopecia. Blood Transfus 21(1): 24-36.
- Arita A, Tobita M (2024) Adverse events related to platelet-rich plasma therapy and future issues to be resolved. Regen Ther 26: 496-501.
- Zeineddine R, Abou Khater D, Mouawad Y, Hamieh C, El-Hussein M (2023) Herpes zoster ophthalmicus (HZO) secondary to platelet-rich plasma (PRP) therapy - A case report. Heliyon. 2023 Dec;9(12): e22815.
- Badran KW, Sand JP. Platelet-Rich Plasma for Hair Loss. Facial Plast Surg Clin N Am. 2018 Nov;26(4):469–85.
- Klifto KM, Othman S, Kovach SJ (2021) Minoxidil, Platelet-Rich Plasma (PRP), or Combined Minoxidil and PRP for Androgenetic Alopecia in Men: A Cost-Effectiveness Markov Decision Analysis of Prospective Studies. Cureus 13(12): e20839.
- Pozo-Pérez L, Tornero-Esteban P, López-Bran E (2024) Clinical and preclinical approach in AGA treatment: a review of current and new therapies in the regenerative field. Stem Cell Res Ther 15(1): 260.
- Nagaja SA, John RS, Kumar SP, Krishnan M (2024) Comparison of the Efficacy Between Regional Nerve Block and Ring Block as Local Anesthetic Techniques for Platelet-Rich Plasma Treatment. Cureus 16(2): e53901.






























