Abstract
Introduction: The widespread use of hyaluronic acid (HA) fillers in aesthetic medicine has resulted in an increase in complications, requiring proper management. Hyaluronidase (HYAL) is the primary treatment for HA-related complications; however, no official guidelines currently exist.
Objective: This consensus aims to establish best practices for HYAL usage and address specific challenges in Italy.
Methods: A group of Italian aesthetic practitioners, plastic surgeons, and dermatologists convened to discuss the use of HYAL. An initial document was reviewed and revised a consensus meeting during the EBAM 2024 annual congress, resulting in final recommendations shared with all participants, who provided additional suggestions that contributed to this final document.
Results: The panel recommends using only pharmacy-sourced galenic HYAL and avoiding non-galenic or cosmetic versions. Due to potential allergic reactions and pH changes affecting its efficacy, HYAL should not be mixed with other drugs such as lidocaine. Routine allergy testing is unnecessary unless there is a history of hypersensitivity. Cold (non-inflammatory) nodules occur from incorrect filler placement, while hot (inflammatory) nodules are linked to immune-mediated reactions. Treatments include HYAL, corticosteroids, and antibiotics. For vascular complications, HYAL should be injected immediately, preferably under ultrasound guidance. In the absence of ultrasound, larger doses are advised. Regarding vision loss, the panel discussed on whether aesthetic doctors should perform retrobulbar hyaluronic acid injections for HA-induced blindness, given the low success rates and high associated risks without reaching a consensus. The dosage of HYAL varies based on the type of complication. Lower doses can be utilized with ultrasound guidance, while larger amounts are necessary when ultrasound is not available. Retreatments for nonvascular complications should be spaced at least 15 days apart.
Conclusion: This consensus, aiming to make the use of HYAL consistent in Italy, may also be more broadly accepted, enhancing safety and effectiveness in addressing HA filler complications. Additional studies are needed to refine these recommendations.
Keywords:Hyaluronic acid; Fillers; Filler’s complications; Hyaluronidase
Introduction
The number of aesthetic medicine treatments, including those involving hyaluronic acid (HA) fillers, has grown notably in recent years [1]. These treatments are highly appreciated for their ability to offer immediate results while being minimally invasive and reversible. However, they may result in complications requiring adequate management. Many publications have provided us with a framework for complications and their treatment by hyaluronidase (HYAL) [2-17], but currently, there are no guidelines regarding the use of HYAL, as was pointed out in a recent publication [18]. Finally, it is also necessary to underline technological innovations such as ultrasound, which offer greater precision and safety in administering HYAL, making intralesional injections possible and, consequently, allowing the use of significantly lower and targeted dosages to manage filler complications [19-21].
Purpose
An Italian consensus document was developed to promote best practices in managing filler complications and administering HYAL.
Method
The chairperson (PM) conducted a thorough review of the existing literature on HYAL, prepared an introductory document for a consensus meeting, and gathered a group of thirteen experienced Italian doctors: seven aesthetic practitioners, five plastic surgeons and one maxillofacial surgeon, and one dermatologist (hereinafter referred to as “this Panel”), with assistance from a lawyer for legal advice. All the experts have many years of experience in treating filler complications. During the 2024 annual EBAM meeting, each topic proposed by the chairperson was thoroughly discussed and integrated with comments from the panel. At the end of the discussion, each member in the panel voted on the proposed recommendation. The consensus was reached when >75% of participants agreed [22]. The discussion, along with all the issues that emerged, and the final recommendations were summarized in a final document. This document was then shared with all participants who contributed to a new version incorporating their last comments and suggestions. The final document was circulated, and the entire panel expressed their agreement.

Topics included in this Consensus document
Hyaluronidase: general framework
HYAL is a set of different enzymes that deploy hyaluronic acid and allow or promote its reabsorption. Various endogenous HYAL have a half-life of two minutes, are inactivated by the liver and kidneys, have an immediate action at the time of release, a duration of effect varying between 24 and 48 hours, and allow the complete restoration of native hyaluronic acid after 48 hours. Differently, exogenous HYAL are derived from mammals (obtained from the testes), hookworms or leeches, and microbes. Currently available HYAL are of either animal origin or human recombinant. Exogenous HYALs, measured in international units (I.U.), have a behavior similar to endogenous HYALs once injected and are rapidly deactivated [23-25]. Unlike in many other countries, Italy does not have a HYAL drug produced by the pharmaceutical industry, and the use of drugs from different countries not registered in Italy is not only impossible but is prosecuted (these drugs can only be used for personal use). In Italy, injectable HYAL is available exclusively as a galenic preparation (law 94/1998) [26,27], meaning a product made in the laboratory of a pharmacy where the active ingredients with excipients are transformed into medications. Galenic HYAL is prepared in liquid or freeze-dried form. When properly stored, galenic HYAL expires in one year. However, according to the mentioned law, every galenic drug must be used within six months of its preparation, even if the expiry date is indicated otherwise on the package leaflet. Other types of HYALs are on the Italian market, not through the pharmaceutical circuit. These HYALs are authorized only for cosmetic use and cannot be used as a drug to treat complications from HA fillers.
Combination of other drugs with hyaluronidase
Hyaluronidase is a drug and, as such, it is advisable that it is injected without adding any other drug to avoid altering its pharmacological properties. Adding lidocaine to reduce its painful effect is a common practice. However, lidocaine changes the pH of the drug, may pose a risk of systemic absorption of the anesthetic with potential complications, and, finally, there is no evidence to support using lidocaine to reduce bruising [28]. If lidocaine must be used, it is suggested that it be administered separately before HYAL. Other drugs that may facilitate the spread of HYAL should also be injected at different times.
Prevention of allergic reactions to hyaluronidase
Allergic reactions to HYAL are primarily reported in the anesthesiology and ophthalmology literature when very high doses (over 200,000 IU) are administered. However, they are infrequent (0.05%-0.69%) in aesthetic literature. Rather than the drug itself, these allergic reactions may result from contaminants or the addition of other drugs. For instance, the benzyl alcohol used as a preservative in the saline solution to reconstitute the lyophilized HYAL has an allergy rate of 1.3%, which is higher than that of HYAL itself [3]. Although an allergy test before HYAL administration is recommended (particularly for individuals with allergies to Hymenoptera and those with deferrable treatment plans), it is not advised when conducted by the aesthetic clinician, according to recommendations from scientific societies in allergy and immunology. The British Society of Allergy and Clinical Immunology (BSACI) states that allergy tests should not be performed to screen for drug allergies without a clinical history of immediate hypersensitivity type 1. Additionally, these tests can be challenging to perform and interpret without considerable experience; allergens are often significantly diluted to prevent false positives, making false positivity a more pressing concern than the benefits of the test. Lastly, a validated guideline regarding the concentration of HYAL to be used is not currently available. In conclusion, patients at risk of severe allergic reactions should be referred to specialist allergy centers [28]. However, the practitioner must be prepared to face an allergic reaction. In particular, an emergency kit, including access to epinephrine, must be available in the clinic, and the staff must be trained to handle emergencies.
Supportive therapies
According to the latest publications [29-31] supportive care is no longer deemed necessary or has not shown tremendous benefit. Nitroglycerin-based ointment, acetylsalicylic acid, and Nonsteroidal Anti-Inflammatory Drugs, which were always administered in the past, are indicated in the most recent recommendations only as accompanying therapies in case of symptoms. Specific therapies should only be used for any clinical picture associated with the main one, such as antivirals in case of Herpes lesions occurrence or antibiotics in case of skin suffering from vesiculation and/or necrosis where a superinfection could develop.
Hyaluronidase reconstitution
Lyophilized HYAL must be reconstructed. It has been suggested that it be reconstructed with saline in a more concentrated form (300 to 1500 IU/ml for treating ischemic lesions) or a more diluted form (150 to 300 IU/ml) for hypercorrection treatment or ultrasound-guided injection). Some also suggest always injecting a large number of solutions in a significantly diluted form to allow them to spread better.
Indications for the use of hyaluronidase
Hyaluronidase has several main indications: cold, non-inflammatory nodules, hot or inflammatory nodules, hypercorrections, and vascular complications.
Nodules
Cold nodules are palpable but not always visible except in areas where the skin is very thin (eyelids, nasolabial region, lips). They are well-demarcated by the surrounding tissues. The causes of their formation can be the injection of an excessive filler amount, an erroneous filler positioning [32] or the incongruence between the rheological characteristics of the filler and their positioning. The formation of nodules can also be facilitated by placing the filler in areas with significant muscle activity, such as the area around the modiolus. Hot or inflammatory nodules recognize a complex and not fully understood granulomatous inflammatory pathophysiology. This is a type IV systemic hypersensitivity reaction. All initially injected sites appear to be adversely affected simultaneously, and all injected implants cause a foreign body reaction [30,33-39]. The hot nodules are characterized by a late onset, appearing weeks or months after treatment and consist of multiple red plaques or nodules, with edema, erythema, and a rigid consistency that can result in fibrosis. In the past, its formation occurred mainly following the injection of a permanent or semi-permanent filler. Currently, granuloma occurrence is more frequent with other types of HA fillers, more persistent and stable due to new laboratory and implant technologies (even if not classified as permanent). Immune-mediated reactions have also increased in severity and incidence due to the COVID-19 pandemic and its vaccines [40].
The treatment of nodules is not simple. Establishing a sequence of interventions is necessary because of the different opinions and attitudes. Initial treatment with massage and saline injection was suggested to distribute the product [29,31,34,38]. When excessive implantation of hyaluronic acid has been superficial, extrusion can be attempted with a large caliber needle (22-18G) followed by HYAL injection to dissolve the residual filler. Treatment with HYAL should be administered if previous procedures have proven ineffective or only partially effective. Intralesional HYAL injections should ideally be performed, as it is generally more effective. There is no consensus on whether local treatment should precede or follow general treatment or whether other medications, such as intralesional corticosteroids or 5-fluorouracil, should also be utilized, particularly in cases involving hot nodules [31,34,37- 39]. There has been a difference in the treatment of cold and hot nodules among experts. The most used drugs to treat cold nodules are HYAL and cortisone. Intralesional injections of these drugs are performed as the first treatment by 43% and 28% of experts, respectively. Other suggested treatments were systemic cortisone administration, antibiotic therapy, surgical evacuation, and intralesional Laser treatment (ILT) [41,42]. For hot nodules, 64% of experts start with antibiotic therapy. Hyaluronidase is used as the first treatment by only one expert. Cortisone is added as a second treatment. Hyaluronidase is associated with previous treatments by 40% of experts and surgical evacuation and ILT by two experts.
Vascular complications
A review of 55 publications [43] points out that major vascular complications are due to an intravascular filler injection and not to vessel compression by a near-the-vessel injection. The necrosis of surrounding and adjacent tissues due to a lack of blood supply results from vessel occlusion. Symptoms and signs of an ischemic event include whitening, paresthesia, cold skin, and pain; however, these signs may not appear immediately and can emerge later. Consequently, they might not be readily observable by the doctor, complicating the understanding of the complication’s occurrence. Timeliness in treatment is crucial; the sooner the intervention, the better the outcome: the complication resolves quickly, with minimal or no signs over time. The use of HYAL is mandatory. When possible, it should be injected into the lesion or the area where the filler was injected as soon as feasible under ultrasound guidance [19-21]. Ultrasound guided injections allow a dramatic reduction of HYAL dosage [19-21]. Extrusion with an 18-gauge needle or scalpel tip to facilitate HA release seems unnecessary since the filler injection was intravascular. Quick differentiation between hematoma and major vascular accidents is also critical. Large hematomas may hide the underlying damage, but they should be treated with substantial amounts of HYAL if an ultrasound machine capable of detecting all obstructions is unavailable.
Hyaluronidase Dosages
There are different indications for administering hyaluronidase depending on many variables: the type of problem we must treat, the type of filler injected, the degree of crosslinking, concentration, the technology used, the density, G’, the implantation depth, the area involved and the anatomical region of filler injection.
Nodules
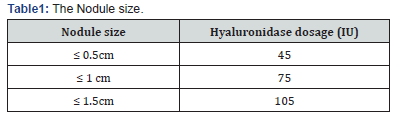
The dosage of hyaluronidase for treating nodules resulting from previous HA filler implants varies in terms of the unit count and volume, influenced by several factors that can affect the outcome. Some dosages have been reported depending on the anatomical regions, 15 nodule size [44] (Table I), the extent of the involved area [45] (Table 2), and the individual authors [46,47] (Table 3).


Tyndall effects and hypercorrection
The literature recommends a single administration of HYAL ranging from 30 to 75 IU [27,48,49], However, there is unanimous agreement that if a satisfactory result of HA degradation has not been achieved, retreatment should not be done before 15 days; some even suggest waiting 30 days. Rapid and close timing for administration should be avoided. For hypercorrection, 5 IU of hyaluronidase degrades 0.1 ml of 20 mg/ml hyaluronic acid unless an ultrasound-guided injection is administered, in which case the dosage is adjusted.
Vascular complications
Traditionally, the dosage of hyaluronidase follows the socalled flooding or washout technique, recommending 450-1500 IU concentrated in 2.5 milliliters of saline solution. As a result, large amounts of hyaluronidase may be repeated for up to four cycles, with each cycle spaced one hour apart. Supplementation with other general and local pharmacological support therapies, such as nitroglycerin administered topically and sublingually, aspirin, and cortisone [50-53], should only be performed if necessary to address any accompanying symptoms.
The issue of the HYAL dosage is made even more complicated
by the availability of ultrasound. The ultrasonographic study offers
several advantages in treating filler complications: identifying
HA accumulation, the vessel and the flow stop in case of vascular
complications, and precise intralesional administration of HYAL.
Indeed, when performed under US guidance, the HYA dosage can
be significantly reduced [19-21], and injecting smaller doses than
the usually recommended is possible. Unfortunately, the use of
ultrasound machines is not widespread now, and few colleagues
can benefit from them. If the ultrasound machine is unavailable,
it will be necessary to proceed with HYAL washout, regardless
of any dose-dependent allergic reactions or post-administration
inflammation/irritation. There was a consensus in pointing out
that
• The amount and timing of administration of HYAL
vary depending on the problem we want to treat (Tyndall effect,
nodules, hypercorrections, vascular complications)
• the amount of HYAL for the treatment of nodules
varies depending on the amount of filler injected, the degree of
crosslinking, the G’ and the site of injection, whether superficial
or deep.
Vision loss due to retinal ischemia
Sudden blindness that occurs during the execution of aesthetic treatment is likely caused by the accidental injection of the filler into the branches of the external carotid artery (supratrochlear AA, supraorbital, angular, and dorsal of the nose). The filler, pushed under high pressure, rises retrograde to the ophthalmic artery or its branches, causing its occlusion [54-56]. The indication is to stop the injection procedure immediately and arrange for the prompt transfer of the patient to a hospital or specialist. In the meantime, alternative treatments have been suggested even if there are no data on their effectiveness: ocular massage to dislodge the embolus, CO2 rebreathing to increase perfusion, timolol drops, intravenous mannitol and acetazolamide to reduce intraocular pressure, and aspirin (300mg) to prevent clot formation. More aggressive treatments include a retro-bulbar or intra-arterial HYAL injection. Treatment should occur as quickly as possible, within minutes of the onset of symptoms, to prevent blindness. Even if there have been rare cases where treatment was administered even after many hours or days, resulting in vision recovery [57,58] prompt intervention remains critical and is the standard recommendation. The HYAL dosage outlined in this indication varies from 450 IU in three close injections to 1800 IU in two close injections. Higher doses, up to 6000 IU, have not proven more effective. The volume to be injected is not specified, but due to the limited capacity of the retrobulbar space, it is advisable to inject concentrated hyaluronidase to avoid causing retrobulbar compartment syndrome, which could potentially worsen the condition.
Results
Regarding the HYAL general framework, this Panel recognized
that HYAL is a drug that exists in Italy only in galenic form and
unanimously recommends:
• It is purchased in pharmacies.
• Only galenic HYAL, whether liquid or freeze-dried, can
be used on the patient.
• Galenic HYAL should be used within six months of its
preparation, even if otherwise indicated by the manufacturer.
• Other HYALs that do not meet the above requirements
are not used for medical purposes.
Regarding the Combination of other drugs with hyaluronidase,
this Panel (unanimously -1) recommends that:
• No drugs should be added to HYAL.
• Medications one wishes to combine should be injected
separately from HYAL.
Regarding the Prevention of allergic reactions to hyaluronidase,
this Panel (unanimously -1) recommends that:
• A hyaluronidase allergy test should not be carried out,
even in case of deferrable treatment
• In case of a severe fear of allergic reactions, the patient
should be referred to specialist allergy centers.
Regarding Supportive therapies, this Panel unanimously recommends that supportive care be avoided and its use be limited to treating concomitant symptoms. Regarding HYAL reconstitution, this Panel unanimously recommends that HYAL be reconstituted with saline at 300-1500 IU/ml dilution in case of ischemia and that dilutions greater than 150-300 IU/ml must be used in case of overcorrection or ultrasound-guided injection.
Regarding the Indications for HYAL use, this Panel unanimously recommends that HYAL should not be used to correct previous non-symptomatic and non-dysmorphic treatments before a new one. No recommendation can be made for treating cold and hot nodules. The Panel suggests preferential use of intralesional HYAL or intralesional or systemic corticosteroid treatment for cold nodules. Antibiotic therapy and systemic cortisone have been suggested as the first treatments for hot nodules. Hyaluronidase can be used in case of failure. Other treatments (surgical excision, ILT) are left to individual judgment and skills. Regarding vascular complications, this panel unanimously recommends that HYAL treatment be administered as promptly as possible. Hyaluronidase should be injected into the lesion under ultrasound guidance. If ultrasound guidance is unavailable, the affected area should be treated with higher amounts of HYAL (washout).
Regarding HYAL dosage, this Panel unanimously recommends
that:
• The dosage of HYAL depends on the filler complication
and whether it is vascular or non-vascular (nodules, Tyndall effect,
hypercorrection), on the characteristics of the injected HA, on the
involved area, and on the anatomical region of injection.
• When treating non-vascular complications, close HYAL
injections should be avoided when the result is unsatisfactory, and
a new treatment should be delayed for 15 to 30 days.
• Hyaluronidase dosage can be reduced with ultrasoundguided
injection, which is recommended over the traditional
administration not ultrasound-guided.
• When ultrasound guidance is unavailable, the area to be
corrected is “flooded” with a higher amount of HYAL (wash-out).
Regarding the vision loss due to hyaluronic acid injection, this Panel unanimously recommends that in the event of vision loss, the injection should be stopped immediately, alternative treatments should be administered, and swift arrangements should be made for the patient’s transfer to a hospital or specialist. This Panel did not reach a consensus on whether the aesthetic doctor or surgeon should acquire the competence to perform the retrobulbar injection (with three experts in favor and ten against), which is left to each person’s judgment and skills.
Discussion
Hyaluronidase continues to be used to address complications in various ways. This practice is not limited to Italy; it is also widespread globally [18]. Many aspects reflecting the diverse uses of hyaluronidase among Italian doctors were thoroughly discussed before reaching a consensus during the meeting. Additionally, the chairperson reported the routine use of hyaluronidase by colleagues not included in the panel, contrasting with this final consensus. In particular, aesthetic practitioners may use nongalenic drugs not supplied by the pharmaceutical circuit, combine hyaluronidase with lidocaine, recommend allergy tests before deferable treatments, apply varying dilutions and dosage for the same complication, adopt different approaches for hot or cold nodules, inject hyaluronidase to reverse prior HA treatments even when complications do not occur, and disagree on retrobulbar hyaluronidase injections in cases of blindness following HA injections. The panel especially focused on common behavior among aesthetic practitioners and surgeons. With the proliferation of aesthetic treatments and the growing number of aesthetic medicine practitioners, patients are accustomed to seeing multiple professionals with various skills and aesthetic preferences. Concurrently, incidents of patients experiencing accumulations and overcorrections of hyaluronic acid and/or other fillers have risen [59-61]. Some practitioners choose to remove previously injected hyaluronic acid, especially in the lips and peri-labial area, before administering new injections. This sometimes occurs when accumulations or hypercorrection result from improper, incorrect, or excessive prior injections. However, the previous HA filler is more often dissolved by some practitioners with HYAL solely or primarily to cater to the aesthetic preferences of the professional or in response to current trends. The panel agreed that HA removal should not be practiced for purely aesthetic reasons, to satisfy the personal preferences of the injecting doctor, or to correct minor imperfections that could be managed with less invasive measures. It should only be performed, when necessary, particularly in cases of hypercorrection or in cases of serious complications like significant asymmetry, the appearance of nodules, or vascular accidents. The indiscriminate application of this technique could lead to further inflammatory issues [49,62- 64] and exaggerate tissue stimulation, while the management of minor aesthetic defects should be approached with more targeted and less invasive corrections.
In many non-controversial situations, discussions led to a broad consensus, although in two cases, only one of the panel experts disagreed. A consensus was not reached regarding the treatment of cold or hot nodules. This panel suggests the most appropriate treatments, but disagreement remains about how the treatments should be sequenced. Unfortunately, there are no clear indications or guidelines sufficiently referencing the treatment of nodules, and the panel’s differing attitudes reflect the various indications found in the literature. Some specific procedures (surgical excision, ILT) depend on individual expertise. A clear indication for the HYAL dosage in different filler complications was not provided. This does not indicate a lack of consensus; on the contrary, the panel agreed that indicating a precise HYAL dose is impossible, as too many variables play a role in deciding the proper dosage. This aligns with the data from the literature, where a precise dosage cannot be found, and significant variability of HYAL dosages (ranging from flooding the area to minimal amounts of HYAL units) has been reported. The panel also agreed that ultrasound guidance can play an important role in reducing the HYAL dosage. Injecting HYAL inside the lesion and immediately visualizing its dissolution allows for the use of minimal HYAL dosage amounts. Lastly, there was complete disagreement regarding the necessity of educational courses for aesthetic practitioners or surgeons to acquire the skills for performing retrobulbar injections. The question arose as to whether we should be able to perform this procedure for a complication that occurs exceptionally, as some propose [65], in the same manner as we must be equipped to carry out cardiopulmonary resuscitation, even if such events are rare. This issue was heavily debated during the consensus meeting with three experts in favor and the remaining against. On one hand, it was emphasized that this is the only treatment that can partially or fully restore the patient’s vision. On the other hand, it was pointed out that this procedure is not risk-free. On the contrary, it can cause additional complications, such as retrobulbar hemorrhage (occurring in 3% of cases), perforation of the sclera (1/5000 cases), retinal vascular occlusion, injection into the optic nerve, compartment syndrome in the retrobulbar space, or optic disc edema when large amounts of fluid are injected [66,67]. In front of all these complications, the positive outcomes of retrobulbar HYAL injection in restoring vision are limited. A recent review indicated that only 3 out of 17 patients regained their vision after retrobulbar HYAL injection.68 Additionally, our lawyer highlighted another important issue. While a doctor may not be deemed responsible for a complication that is well described to the patients and after achieving their informed consent, they can be liable for additional damage to the patient. In conclusion, most experts (10 out of 13) favor promptly transferring the patient to a hospital or specialist with the necessary skills. In the meantime, the described alternative procedures are recommended. Finally, it is advised that those who perform this procedure must be able to carry it out safely.
The value of this consensus lies in the common fears and perplexities expressed by the involved experts, which also stem from the literature regarding HYAL use. The issues of storage and reconstitution, the combination with lidocaine, fears of allergic reactions and the need for allergy tests, concerns over long-term effects, the time interval between treatments, and the varying dosages to treat nodules or vascular complications-raised and debated in our consensus meeting-reflect existing literature. We believe that, even if limited to a small group of Italian experts, the recommendations and suggestions of this consensus can be widely shared and applied. Study limitations: the main study’s limitation is the small number of experts included in the panel compared to the large number of doctors and surgeons involved in aesthetic medicine in Italy. However, all of them are well-known Italian experts with many years of experience, which provides them with significant expertise in handling complications and their treatment. Nonetheless, future studies involving a much larger number of aesthetic medicine practitioners are recommended to confirm the outcomes of this consensus. Finally, we do not think that the lack of consensus in providing the readers with a precise algorithm or flowchart in treating filler complications may be considered a limit of this work, as this reflects the uncertainties coming from literature. At least we raised the point and proposed solutions, even if with some discrepancies. Also, the lack of consensus regarding retrobulbar HYAL injection cannot be considered a study limit. Indeed, no consensus exists worldwide regarding these two items. Some could consider a limitation of the study of the adopted consensus method. However, we used a method widely adopted in other consensus documents. In addition, we want to highlight that several approaches exist for determining consensus, each with some criticisms. Consequently, an ideal methodology for determining consensus has not yet been established. In a relatively recent paper [69] several methods are listed, and the authors conclude that more action must be taken to establish the optimal method. In a few years, a critical analysis by AI of the various methodologies will likely lead to a better proposal for a more specific tool for consensus.
Conclusions
In the absence of international guidelines, the use of hyaluronidase is still different among aesthetic medicine practitioners and plastic surgeons. This lack of uniformity is common worldwide. We believe that this consensus document may contribute to making the treatment of complications by HYAL more consistent, at least on some very debated aspects.
References
- Gotadki R (Dermal Fillers Market Research Report Information by Type (Hyaluronic Acid, Calcium Hydroxylapatite, Poly-L-Lactic Acid, and Others) by Application (Facial Line Correction, Face lift, Lip Enhancement, and Others), by End User (Specialty & Dermatology Clinics, Hospitals, and Others), by Region (North America, Europe, Asia-Pacific, and Rest of the World) - Forecast till 2032.
- DeLorenzi C (2017) New high dose pulsed hyaluronidase protocol for hyaluronic acid filler vascular adverse events. Aesthet Surg J 37(7): 814-825.
- Murray G, Convery C, Walker L, Davies E (2021) Guideline for the safe use of hyaluronidase in aesthetic medicine, including modified high-dose protocol. J Clin Aesthet Dermatol 14(8): E69-E75.
- Ors S (2020) The effect of hyaluronidase on depth of necrosis in hyaluronic acid filling-related skin complications. Aesthetic Plast Surg 44(5): 1778-1785.
- Rivers JK (2022) Incidence and treatment of delayed-onset nodules after VYC filler injections to 2139 patients at a single Canadian clinic. J Cosmet Dermatol 21(6): 2379-2386
- King M, Bassett S, Davies E, King S (2016) Management of delayed onset nodules. J Clin Aesthet Dermatol 9(11): E1-E5.
- Chung KL, Convery C, Ejikeme I, Ghanem AM (2020) A systematic review of the literature of delayed inflammatory reactions after hyaluronic acid filler injection to estimate the incidence of delayed type hypersensitivity reaction. Aesthet Surg J 40(5): 286-300.
- Bhojani-Lynch T (2017) Late-onset inflammatory response to hyaluronic acid dermal fillers. Plast Reconstr Surg Glob Open 5(12): e1532.
- Lee JM, Kim YJ (2015) Foreign body granulomas after the use of dermal fillers: pathophysiology, clinical appearance, histologic features, and treatment. Arch Plast Surg 42(2): 232-239.
- Convery C, Davies E, Murray G, Walker L (2021) Delayed-onset nodules (dons) and considering their treatment following use of hyaluronic acid (ha) fillers. J Clin Aesthet Dermatol 14(7): E59-E67.
- Jung H (2020) Hyaluronidase: an overview of its properties, applications, and side effects. Arch Plast Surg 47(4): 297-300.
- Dunn AL, Heavner JE, Racz G, Day M (2010) Hyaluronidase: a review of approved formulations, indications and off-label use in chronic pain management. Expert Opin Biol Ther 10(1): 127-131.
- Borzabadi-Farahani A, Mosahebi A, Zargaran D (2024) A Scoping Review of Hyaluronidase Use in Managing the Complications of Aesthetic Interventions. Aesthetic Plast Surg 48(6): 1193-1209.
- Bailey SH, Fagien S, Rohrich RJ (2018) Changing role of hyaluronidase in plastic surgery. Plast Reconstr Surg 133(2): 127e-132e.
- King M, Convery C, Davies E (2018) This month’s guideline: The Use of Hyaluronidase in Aesthetic Practice (v2.4) J Clin Aesthet Dermatol 11(6): E61-E68.
- Kapoor KM, Kapoor P, Heydenrych I, Bertossi D (2020) Vision loss associated with hyaluronic acid fillers: a systematic review of literature. Aesthetic Plast Surg 44(3): 929-944.
- Zheng C, Fu Q, Zhou GW, Lai LY, Zhang LX, et al. (2022) Efficacy of percutaneous intraarterial facial/supratrochlear arterial hyaluronidase injection for treatment of vascular embolism resulting from hyaluronic acid filler cosmetic injection. Aesthet Surg J 42: 649-655.
- Currie E, Granata B, Goodman G, Rudd A, Wallace K, et al. (2024) The Use of Hyaluronidase in Aesthetic Practice: A Comparative Study of Practitioner Usage in Elective and Emergency Situations. Aesthet Surg J44(6): 647-657.
- Quezada-Gaón N, Wortsman X (2016) Ultrasound-guided hyaluronidase injection in cosmetic complications. J Eur Acad Dermatol Venereol 30(10): e39-e40.
- Schelke LW, Velthuis PJ, Decates T, Kadouch J, Alfertshofer M, et al. (2023) Ultrasound-Guided Targeted vs Regional Flooding: A Comparative Study for Improving the Clinical Outcome in Soft Tissue Filler Vascular Adverse Event Management. Aesthetic Surgery Journal. Aesthet Surg J 43(1): 86-96.
- Urso SU, Molinari P, Fundarò P, Mosti G (2014) Use of Minimal Amounts of Hyaluronidase in the Ultrasound Guided Treatment of Acute Vascular Occlusion by Hyaluronic Acid: A Preliminary Report. Aesthetic Surgery Journal Open Forum 6: ojae025.
- Diamond IR, Grant RC, Feldman BM, Pencharz PB, Ling SC, et al. (2014) Defining consensus: a systematic review recommends methodologic criteria for reporting of Delphi studies. J Clin Epidemiol 67(4): 401-409.
- De Almeida ART, Saliba A. Hyaluronidase in cosmiatry: What should we know? Surgical & Cosmetic Dermatology 7(3).
- Lee A, Grummer SE, Kriegel D, Marmur E (2010) Hyaluronidase. Dermatol Surg 36(7): 1071-1077.
- Jung H (2020) Hyaluronidase: An overview of its properties, applications, and side effects. 0, Arch Plast Surg 47(4): 297-300.
- Legge 8 aprile 1998, n94.
- Kroumpouzos G, Treacy P (2024) Hyaluronidase for Dermal Filler Complications: Review of Applications and Dosage Recommendations. JMIR Dermatol 7: e50403.
- Kraft MT, Kraft CT (2024) Management of Hyaluronidase Allergies: The Importance of Specialist Evaluation. Aesthet Surg J 44(11): NP850-NP851.
- Snozzi P, van Loghem JAJ (2018) Complication Management following Rejuvenation Procedures with Hyaluronic Acid Fillers-an Algorithm-based Approach. Plast Reconstr Surg Glob Open 6(12): e2061.
- Sito G, Manzoni V, Sommariva R (2019) Vascular Complications after filler injection: A literature review and Meta-Analysis. JCAD 12(6): E65-E72.
- Kim JH, Ahn DK, Jeong HS, Suh IS (2014) Treatment Algorithm of Complications after Filler Injection: Based on Wound Healing Process. Journal of Korean medical science 29(Suppl 3) : S176-S182.
- Schelke LW, Decates TS, Cartier H, Cotofana S, Velthuis PJ (2023) Investigating the Anatomic Location of Soft Tissue Fillers in Noninflammatory Nodule Formation: An Ultrasound-Imaging-Based Analysis. Dermatol Surg 49(6): 588-595.
- Wojciech M, Olszański R, Sierdzinski J, Ostrowski T, Szyller K, et al. (2019) Treatment of late bacterial infections resulting from soft-tissue filler injections. Infection and Drug Resistance Journal 12: 469-480.
- De Boulle K, Heydenrych I (2015) Patient factors influencing dermal filler complications: prevention, assessment, and treatment. Clin Cosmet Investig Dermatol 8: 205-214.
- Funt D, Pavicic T (2013) Dermal fillers in aesthetics: an overview of adverse events and treatments approaches. Clinical, Cosmetic and Investigational Dermatology 6: 295-316.
- Baranska-Rybak W, Lajo-Plaza JV, Walker L, Alizadeh N (2024) Late-Onset Reactions after Hyaluronic Acid Dermal Fillers: A Consensus Recommendation on Etiology, Prevention and Management. Dermatology and Therapy 14(7): 1767-1785.
- Ibrahim O, Overman J, Arndt KA, Dover JS (2018) Filler Nodules: Inflammatory or Infectious? A Review of Biofilms and Their Implications on Clinical Practice. Dermatol Surg 44(1): 53-60.
- DeLorenzi C (2013) Complications of Injectable Fillers, Part I. Aesthetic Surgery Journal 33(4): 561-575.
- Beleznay K, Carruthers JD, Carruthers A, Mummert ME, Humphrey S (2015) Delayed-onset nodules secondary to a smooth cohesive 20 mg/mL hyaluronic acid filler: cause and management. Dermatol Surg 41(8): 929-939.
- Beamish IV, Bogoch II, Carr D (2022) Delayed inflammatory reaction to dermal fillers after COVID-19 vaccination: a case report. Canadian Journal of Emergency Medicine 24(4): 444-446.
- Schelke LW, Decates TS, van der Lugt CIM, Pelzer L, de Mey G, et al. (2018) Intralesional Laser Treatment for Dermal Filler Complications. Plast Reconstr Surg 141(6): 1361-1369.
- Zaccaria G, Cassuto D, Baccarani A, Lusetti IL, Santis G (2022) Filler-induced complications of the lips: 10 years’ experience with intralesional laser treatment and refinements. J Plast Reconstr Aesthet Surg 75(3): 1215-1223.
- Abduljabbar MH, Basendwh MA (2016) Complications of hyaluronic acid fillers and their managements. Journal of Dermatology & Dermatologic Surgery 20(2): 100-106.
- Marusza W, Olszanski R, Sierdzinski J, Ostrowski T, Szyller K, et al. (2019) Treatment of late bacterial infections resulting from soft-tissue filler injections. Infect Drug Resist 12: 469-480.
- Signorini M, Liew S, Sundaram H, De Boulle KL, Goodman GJ, et al. (2016) Global Aesthetics Consensus: Avoidance and Management of Complications from Hyaluronic Acid Fillers-Evidence- and Opinion-Based Review and Consensus Recommendations. Plast Reconstr Surg 137(6): 961e-971e.
- DeLorenzi C, Karpova E, Rzany B, Trévidic P, Lamilla GC: Anatomy & Filler Complications. E2e, 1 janv. 2017.
- Park KE, Mehta P, Kherani F, Lee WW, Woodward JA, et al. (2023) Response of 21 Hyaluronic Acid Fillers to Recombinant Human Hyaluronidase. Plast Reconstr Surg Glob Open 11(12): e5457.
- King M (2016) Management of Tyndall effect. J Clin Aesthet Dermatol 9(11): E6-E8.
- Olayia OR, Forbes D, Humphrey S, Beleznay K, Mosher M, et al. (2022) Hyaluronidase for treating complications related to HA fillers: a national plastic surgeon survey. Plast Surg (Oakv) 30(3): 233-237.
- DeLorenzi C (2014) Complications of injectable fillers, part 2: vascular complications. Aesthet Surg J 34: 584-600.
- Buhren BA, Schrumpf H, Hoff NP, Bölke E, Hilton S, et al. (2016) Hyaluronidase: from clinical applications to molecular and cellular mechanisms. Eur J Med Res 21: 5.
- Landau M (2015) Hyaluronidase Caveats in Treating Filler Complications. Dermatol Surg 41(Suppl 1): S347-353.
- Cohen JL, Biesman BS, Dayan SH, DeLorenzi C, Lambros VS, et al. (2015) Treatment of Hyaluronic Acid Filler-Induced Impending Necrosis with Hyaluronidase: Consensus Recommendations. Aesthet Surg J 35: 844-849.
- Carruthers JD, Fagien S, Rohrich RJ, Weinkle S, Carruthers A (2014) Blindness caused by cosmetic filler injection: a review of cause and therapy. Plast. Reconstr. Surg 134(6): 1197-1201.
- Carle MV, Roe RH, Novack, RL (2015) Occlusion caused by cosmetic facial filler injection – reply. JAMA Ophthalmol 133(2): 225.
- Kim SN, Byun DS, Park JH, Han SW, Baik JS, et al. (2014) Panophthalmoplegia and vision loss after cosmetic nasal dorsum injection. J. Clin. Neurosci 21(4): 678-680.
- Doyon VC, Liu C, Fitzgerald R, Humphrey S, Jones D, et al. (2024) Update on Blindness from Filler: Review of Prognostic Factors, Management Approaches, and a Century of Published Cases. Aesthet Surg J 44(10): 1091-1104.
- Nguyen HH, Tran HTT, Duong QH, Nguyen MD, Dao HX, et al. (2022) Significant Vision Recovery from Filler-Induced Complete Blindness with Combined Intra-Arterial Injection of Hyaluronidase and Thrombolytic Agents. Aesthetic Plast Surg 46: 907-911.
- Lim T (2024) Facial Overfilled Syndrome. Dermatol Clin 42(1): 121-128.
- Lim TS, Wanitphakdeedecha R, Yi KH (2024) Exploring facial overfilled syndrome from the perspective of anatomy and the mismatched delivery of fillers. J Cosmet Dermatol 23(6): 1964-1968.
- Schelke L, Harris S, Cartier H, Alfertshofer M, Doestzada M, et al. (2023) Treating facial overfilled syndrome with impaired facial expression-Presenting clinical experience with ultrasound imaging. J Cosmet Dermatol 22(12): 3252-3260.
- Shelke LW, Farber N, Swift A (2022) Ultrasound as an educational tool in facial aesthetic injections. Plast Reconstr Surg Glob Open 10(12): e4639.
- Casabona G, Marchese PB, Montes JR, Hornfeldt CS (2018) Durability, behavior and tolerability of 5 hyaluronidase products. Dermatol Surg 44(Suppl 1): S42-S50.
- Kim JH, Kwon SB, Whang KU, Lee JS, Park YL, et al. (2018) The duration of hyaluronidase and optimal timing of hyaluronic acid (HA) filler reinjection after hyaluronidase injection. J Cosmet Laser Ther 20(1): 52-57.
- Prado G, Rodríguez-Feliz J (2017) Ocular Pain and Impending Blindness During Facial Cosmetic Injections: Is Your Office Prepared? Aesthetic Plast Surg 41(1): 199-203.
- Paap MK, Milman T, Ugradar S, Goldberg R, Silkiss RZ (2020) Examining the Role of Retrobulbar Hyaluronidase in Reversing Filler-Induced Blindness: A Systematic Review. Ophthalmic Plast Reconstr Surg 36(3): 231-238.
- Surek CC, Said SA, Perry JD, Zins JE (2019) Retrobulbar Injection for Hyaluronic Acid Gel Filler-Induced Blindness: A Review of Efficacy and Technique. Aesthetic Plast Surg 43: 1034-1040.
- Navarro-Hernandez E, Pérez-López M (2022) Effectiveness of retrobulbar hyaluronidase in the treatment of visual loss caused by periocular hyaluronic acid injection. A systematic review. Arch Soc Esp Ophthalmol (Engl Ed) 97(9): 521-538.
- Van Zuuren EJ, Logullo P, Price A, Fedorowicz Z, Hughes EL, et al. (2022) Existing guidance on reporting of consensus methodology: a systematic review to inform ACCORD guideline development. BMJ Open 12(9): e065154.






























