Compatibility of Commonly Used Active Pharmaceutical Ingredients in Fagron Advanced Derma Dermatological Vehicles
Nina Omerovic, Savvas Koulouridas and Hudson Polonini*
Fagron BV , Fascinatio Boulevard 350, 3065 WB Rotterdam, The Netherlands
Submission: June 19, 2024; Published: July 04, 2024
*Corresponding author: Hudson Polonini, Fagron BV, Fascinatio Boulevard 350, 3065 WB Rotterdam, The Netherlands, Email: Hudson.Polonini@fagron.com
How to cite this article: Nina Omerovic, Savvas Koulouridas and Hudson Polonini*. Compatibility of Commonly Used Active Pharmaceutical Ingredients in Fagron Advanced Derma Dermatological Vehicles. JOJ Dermatol & Cosmet. 2024; 6(2): 555682. DOI: 10.19080/JOJDC.2024.06.555682
Abstract
Background: Inflammatory skin conditions, such as eczema, acne, psoriasis, rosacea, and dermatitis, cause redness, swelling, and itching, affecting both physical and mental well-being. Effective management of these symptoms requires targeted therapeutic interventions. In topical formulations, the choice of vehicle and its compatibility with active pharmaceutical ingredients (APIs) significantly impact treatment outcomes. Emolivan™, Nourisil™, Nourivan™ Antiox, Occluvan™, and Seraqua™ are ready-to-use vehicles developed for compounding pharmacies, formulated with non-irritating ingredients suitable for dermatological applications.
Objective: This study aimed to demonstrate the compatibility of nine commonly used APIs in topical formulations with five ready-to-use dermatological vehicles. The APIs were evaluated for their beyond-use dates at 30, 60, and 90 days, aiming to extend their stability, effectiveness, and safety over an extended period, thereby providing significant convenience for both compounding pharmacists and patients.
Methods: High-Performance Liquid Chromatography (HPLC) analyses were conducted to evaluate the following API concentrations: ascorbic acid 15.0% in Nourivan™ Antiox, clobetasol propionate 0.05% in Occluvan™, desoximetasone 0.25% in Emolivan™, dexpanthenol 5.0% in Nourisil™, hyaluronic acid 2.5% in Nourisil™, resorcinol 15.0% in Nourivan™ Antiox, retinol 0.40% in Seraqua™, salicylic acid 20.0% in Occluvan™, and urea 10.0% in Emolivan™.
Results: All nine APIs demonstrated compatibility with their respective vehicles for at least 60-90 days.
Conclusion: This study validates the stability of nine APIs from different pharmacological classes with Emolivan™, Nourisil™, Nourivan™ Antiox, Occluvan™, and Seraqua™. These findings suggest that these ready-to-use vehicles can be viable options for personalized treatments of inflammatory skin conditions. The insights gained from this research can inform the development of optimized dermatological formulations with enhanced stability, efficacy, and patient acceptability.
Keywords: Dermatological applications; Vehicles; Compounding; Personalized medicine; Active pharmaceutical ingredients
Abbreviation: APIs: Active Pharmaceutical Ingredients; SLS: Sodium Lauryl Sulfate
Introduction
Compounding vehicles are fundamental in dermatological treatments. They act as carriers for active pharmaceutical ingredients (APIs) to ensure effective delivery to the skin [1]. The vehicle choice directly impacts the formulation’s stability, efficacy, and patient acceptance [2]. This is particularly crucial when addressing inflammatory skin conditions, such as eczema, acne, psoriasis, rosacea, dermatitis, and premature aging, which affect 20-25% of the population [3]. These conditions, mainly when localized on visible areas like the face or exposed body parts, can profoundly impact both the physiological and psychological well-being of patients [4,5]. Consequently, a comprehensive treatment approach that targets symptoms while considering patient preferences is necessary.
Patient perception is a critical yet frequently overlooked aspect of dermatological treatments. With increasing access to resources on skincare regimens, patients are more likely to seek products that align with their health values and concerns. This growing consciousness underscores the importance of transparency and safety in formulating dermatological vehicles. Features such as greasiness, ease of application, and residue can influence a patient’s willingness to use a product, affecting treatment adherence and outcomes [6,7]. Additionally, heightened awareness of controversial ingredients, such as parabens and synthetic fragrances, has made patients more vigilant about the components in their skincare regimens [8]. To address these factors effectively, it is essential to formulate and recommend vehicles that optimize the performance of APIs. The vehicle plays a crucial role in compatibility with the patient’s skin type and stability when delivering a particular API [9]. Factors such as texture, absorption rate, and compatibility with the skin influence the choice of the vehicle, ensuring both therapeutic efficacy and patient acceptance. Equally important is the careful selection of APIs based on their mechanism of action for the specific pathology and patient profile. This compatibility study focuses on a select group of APIs commonly used in dermatological treatments for inflammatory skin conditions. These APIs were chosen based on their widespread use, therapeutic relevance, and varying physicochemical properties, providing a comprehensive basis for evaluating vehicle compatibility. In this study, ascorbic acid, clobetasol propionate, desoximetasone, dexpanthenol, hyaluronic acid, retinol, resorcinol, salicylic acid, urea was selected as APIs. These APIs, utilized for various dermatological conditions, were selected for compatibility testing to further increase the utility of Emolivan™, Nourisil™, Nourivan™ Antiox, Occluvan™, and Seraqua™ as vehicles with adequate stability. In doing so, we contribute to advancing the field of dermatology by fostering innovations that are scientifically backed while at the same time patient centered.
Materials and Methods
Reagents, Reference Standards, and Materials
Fagron supplied all the APIs for this study. Methanol and acetonitrile were supplied by Merck; ethyl-4-hydroxybenzoate by Aldrich; ethanol 96%, propanol-2, orthophosphoric acid 85%, sodium hydroxide 1M, sulfuric acid 1N, chloroform and sodium metabisulfite from VWR; acetic acid, glycerol, butylated hydroxytoluene, potassium phosphate monobasic, sodium phosphate dibasic dodecahydrate, sodium phosphate monobasic monohydrate from Sigma; pentane, potassium hydroxide 38%, orthophosphoric acid 85% from Prolabo; tetrabutylammonium hydroxide in 2-propanol from Carlo Erba; benzophenone from Fluka. All the vehicles, Emolivan™, Nourisil™, Nourivan™ Antiox, Occluvan™, and Seraqua™, were supplied by Fagron. Lastly, all volumetric glassware and the analytical balance used were calibrated.
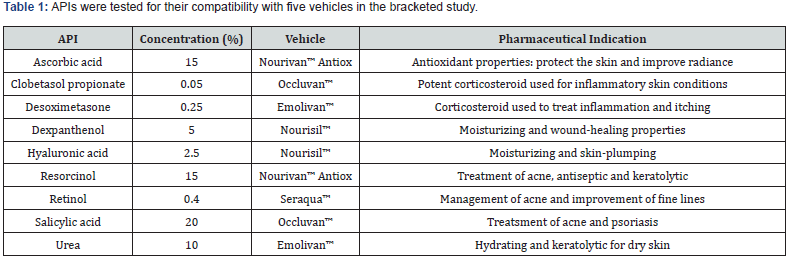
The concentrations of the nine APIs and their intended uses are listed in Table 1.
Equipment
HPLC analyses were performed on a qualified and calibrated chromatography system. This system was composed of a quaternary gradient pump (G1311B), ALS (G1329B), TCC (G1316A), DAD (54212B), VWD (G1314F), and the Isopump (G1310B) from by Agilent; UV detector (L-2400), Autosampler (L- 2200), and Column oven (L-2350) from Hitachi.
Chromatographic Conditions
The chromatographic determinations were developed inhouse. The exact chromatographic conditions used for each API are stated in Table 2. The columns related to a pre-column with the same packing (4.0 × 3.0 mm, 5 μm) from the same vendor as the columns.
Validation of the High-Performance Liquid Chromatography Method
The HPLC methods mentioned in this study were validated following internal protocols aligned with the USP and the ICH guidelines [10]. To assess the specificity of each method, comparisons were made using HPLC analyses of a standard solution, a blank Seraqua™, Occluvan™, Nourisil™, Nourivan™ Antiox, or Emolivan™ solution, and a blank solution of the mobile phase/diluents, both with and without the matrix. The acceptance criterion for the non-interference of extraneous peaks to the main peak of the API.

Preparation of the Creams
Depending on the vehicle, the samples containing APIs were prepared using the following standardized procedures:
Ascorbic Acid 15.0% in Nourivan™ Antiox
i. Calculate the required quantities of ascorbic acid (15g), purified water (q.s.), and Nourivan™ Antiox (q.s.). Accurately weigh or measure each ingredient.
ii. Combine ascorbic acid and purified water in a ceramic mortar to form a smooth paste.
iii. Using geometric dilution, incorporate Nourivan™™ Antiox into the ascorbic acid paste, mixing well after each addition to form a homogeneous mixture.
Clobetasol Propionate 0.05% in Occluvan™
i. Combine clobetasol propionate and glycerol in a ceramic mortar to form a smooth paste.
ii. Using geometric dilution, incorporate Occluvan™™ into the clobetasol propionate paste, mixing well after each addition to form a homogeneous mixture.
Desoximetasone 0.25% in Emolivan™
i. Combine desoximetasone and sufficient glycerol in a ceramic mortar to form a smooth paste.
ii. Using geometric dilution, incorporate Emolivan™™ into the desoximetasone paste, mixing well after each addition to form a homogeneous mixture.
Dexpanthenol 5.0% in Nourisil™
i. Combine dexpanthenol (5g) with a small amount of Nourisil™™ in a ceramic mortar to form a smooth paste.
ii. Using geometric dilution, incorporate Nourisil™™ into the preparation, mixing well after each addition to form a homogeneous mixture.
Hyaluronic Acid 2.5% in Nourisil™
i. Combine hyaluronic acid (2.5g) and glycerol (q.s.) in a ceramic mortar to form a smooth paste.
ii. Using geometric dilution, incorporate Nourisil™™ into the hyaluronic acid paste, mixing well after each addition to form a homogeneous mixture.
Resorcinol 15.0% in Nourivan™ Antiox
i. Combine resorcinol (15g) with a small amount of Nourivan™™ Antiox in a ceramic mortar to form a smooth paste.
ii. Using geometric dilution, incorporate Nourivan™™ Antiox into the preparation, mixing well after each addition to form a homogeneous mixture.
Retinol 0.40% in Seraqua™
i. Combine retinol, 5.0% propanediol, and a small amount of Seraqua™™ in an appropriately sized FagronLab unguator EMP jar (FagronLab, Scheßlitz, Germany), then mix for 1-2 minutes at a low speed.
ii. Add enough Seraqua™™ to reach the final weight, then mix for 2-3 minutes at medium speed.
iii. If necessary, process the mixture through a Three Roll/ Ointment Mill (FagronLab, Scheßlitz, Germany).
iv. Mix the compound again for 1 minute at low speed or by hand.
Salicylic Acid 20.0% in Occluvan™
i. Combine salicylic acid (20g) and glycerol (q.s.) in a ceramic mortar to form a smooth paste.
ii. Using geometric dilution, incorporate Occluvan™™ into the salicylic acid paste, mixing well after each addition to form a homogeneous mixture.
Urea 10.0% in Emolivan™
i. Combine urea (10g) and purified water (q.s.) in a ceramic mortar to form a smooth paste.
ii. Using geometric dilution, incorporate Emolivan™™ into the urea paste, mixing well after each addition to form a homogeneous mixture.
Stability Study
The cream samples were assayed by HPLC at pre-determined time points to verify the stability of the APIs with their respective vehicles (Table 1). The sampling times were 0 days (t = 0), 30 days (t = 30), 60 days (t = 60), and 90 days (t = 90). A visual inspection was performed to check for phase separation, sedimentation, and discoloration. All solutions were assayed six times, and the results were expressed as the mean from six independent measurements. For that purpose, samples were diluted, sonicated for 10 minutes, and then filtered in 15-mm regenerated cellulose syringe filters with 0.45-μm pore size before injection into the HPLC system. The evaluation parameter was the percent recovery with respect to t =0, using the HPLC method (results given as percentage).
Results and Discussion
Validation studies of all analytical methods were conducted, and the results in Table 3 met the respective acceptance criteria, confirming the methods’ suitability for the study’s objectives. Stability-indicating studies were performed to ensure the methods were fully validated and capable of identifying the decomposition of the APIs through chromatographic analysis. Extraneous peaks were monitored to confirm the absence of late elution peaks, which could indicate impurities or degradation products. At each sampling time, the visual appearance of the solutions was evaluated to verify their homogeneity and physical stability (data not shown). The study observed no phenomena such as sedimentation, discoloration, or phase separation when the API content was within specifications.

Table 4 presents the chemical stability results as a relative percentage of recovery, with the initial sampling time set at 100%. For the creams to be considered stable, the relative percentage recovery should remain within the range of 90% to 110%. This range ensures that the APIs maintain their potency and effectiveness over the specified period, validating the compatibility and stability of the formulations with the selected vehicles. The Fagron Advanced Derma vehicles were designed according to the latest scientific insights into the safety and tolerance of topically applied ingredients. These vehicles are formulated without parabens, boric acid, ethoxylates, 1,4-dioxane, formaldehyde donors, mineral oils, propylene glycol, benzyl alcohol and benzyl benzoate, peanut oil, fragrances and dyes, triclosan, lanolin (wool fat), phthalates, para-aminobenzoic acid, sodium lauryl sulfate (SLS), and nitrosamines, making them suitable for treating sensitive skin due to inflammatory conditions. The study evaluated five different vehicles for their compatibility with various APIs. Emolivan™, a natural W/O cold cream base containing a natural oil-based emulsifier, was used to suit the needs of dehydrated and affected skin due to its protection and hydration effects. Nourisil™, an ultra-light, transparent, self-drying silicone gel, was developed to prevent and manage keloids and hypertrophic scars, maintaining the skin’s moisture balance while improving the appearance of scars with its blend of five silicones and vitamin E. Nourivan™ Antiox, an antioxidant-enriched protective O/W cream, was designed to be the ideal compounding vehicle for APIs susceptible to oxidation, providing balanced hydration. Occluvan™, a hydrophilic ointment base, offered occlusion and hydration effects with its innovative formulation, while Seraqua™, an ultra-light O/W serum base, was designed for facial applications with an elegant formulation. The compatibility of ascorbic acid in Nourivan™ Antiox was validated over 60 days, maintaining the API within 90-110% of its initial concentration. However, the formulation turned yellow and heterogeneous, indicating degradation. This aligns with literature noting the instability of ascorbic acid due to its susceptibility to oxidation when exposed to air and high temperatures. The limitation of ascorbic acid is that, despite maintaining potency, its instability is reflected in discoloration and heterogeneity. The study validated the stability of clobetasol propionate in Occluvan™ over 90 days. This expands the limited research on clobetasol propionate-based topical formulations for dermatological applications, marking the first report to demonstrate its stability in a topical formulation.

Desoximetasone showed stability in Emolivan™ over 90 days, with findings supported by literature indicating that desoximetasone formulations remain stable in skincare applications. Similarly, the compatibility of dexpanthenol in Nourisil™ was validated over 90 days, ensuring its stability and effectiveness for extended use [13]. The compatibility of dexpanthenol over 90 days with the vehicle Nourisil™ has been validated. Hyaluronic acid remained stable in Nourisil™ for 90 days. This aligns with well-documented literature demonstrating the stability of hyaluronic acid-based creams, which increase dermal absorption and ensure maximum effect [14]. Retinol demonstrated compatibility with Seraqua™ over 60 days, maintaining a slightly yellow but homogeneous appearance, which is typical for retinol formulations due to its inherent color. However, the retinol content declined to 83% over 90 days, consistent with its known instability when exposed to light, air, and temperature variations [15,16]. The compatibility of resorcinol in Nourivan™ Antiox was validated over 90 days. Previous research supports that O/W creams protect resorcinol from oxidation and facilitate its microbiological stability. These findings highlight the importance of stabilizers, emulsifiers, and pH control in ensuring long-term stability [17].
Salicylic acid remained stable in Occluvan™ for up to 90 days, supported by numerous studies indicating its stability in properly formulated creams. The stability of urea in Emolivan™ was validated over 90 days, maintaining its concentration within the acceptable range. Limited literature exists on the beyonduse dates of urea in creams, but this study confirms its stability when optimal conditions are maintained [18,19]. The stability of urea in Emolivan™ was validated over 90 days, maintaining its concentration within the acceptable range. Limited literature exists on the beyond-use dates of urea in creams, but Panyachariwat and collaborators (2014) noted that urea stability is directly influenced by temperature and pH. This study confirms that with optimal conditions, urea can effectively enhance skin hydration and barrier function over time, highlighting the significance of stabilizers and proper storage conditions [20].
Conclusion
This compatibility study aimed to demonstrate the effectiveness of Fagron Advanced Derma vehicles with a select group of active pharmaceutical ingredients (APIs) commonly used in dermatological treatments. The goal of this testing was to provide robust data to support patient-centered and personalized therapeutic solutions. Our results indicate that nine dermatologically relevant APIs exhibited compatibility over 90 days (or 60 days for retinol 0.4% in Seraqua™ and acorbic acid 15% in Nourivan™ Antiox) with the respective vehicles from the Fagron Advanced Derma line. Consequently, Fagron Advanced Derma vehicles are demonstrated to be suitable for compounding with a wide range of APIs commonly used in dermatological applications, offering stable and effective treatment options.
References
- Roberts MS, Hanumanth SC, Sean EM, Azadeh A, Heather AE Benson, et al. (2021) Topical drug delivery: History, percutaneous absorption, and product development. Adv Drug Deliv Rev 177: 113929.
- Teixeira A, Maribel T, Vera A, Rita G, Tiago T, et al. (2021) Does the vehicle matter? Real-world evidence on adherence to topical treatment in psoriasis. Pharmaceutics 13(10): 1539.
- Choy SP, Byung JK, Alexandra P, Wei RT, Sarah Man LL, et al. (2023) Systematic review of deep learning image analyses for the diagnosis and monitoring of skin disease. NPJ Digit Med 6: 180.
- Yew YW, Yu Kuan AH, Ge L, Yap CW, Heng BH (2020) Psychosocial impact of skin diseases: A population-based study. Plos One 15(12): e0244765.
- Yang C, Kourosh A (2017) Inflammatory skin disorders and self-esteem. Int J Womens Dermatol 4(1): 23-26.
- Mathias TS, Steven RF, Sylvia NT, Anne Sofie SS, Cecilie Marie RR, et al. (2021) Psoriasis patient preferences for topical drugs: a systematic review. J Derma Treatment 32(5): 478-483.
- Barnes TM, Mijaljica D, Townley JP, Spada F, Harrison IP (2021) Vehicles for Drug Delivery and Cosmetic Moisturizers: Review and Comparison. Pharmaceutics 13(12): 2012.
- Lee YB, et al. (2020) Perceptions and behavior regarding skin health and skin care products: Analysis of the questionnaires for the visitors of skin health expo 2018. Ann Dermatol 32(5): 375-382.
- Torres A, Almeida IF, Oliveira R (2024) An Overview of Proprietary Vehicles/Bases for Topical Compounding Medicines and Cosmetics. Cosmetics 11(1): 16.
- Guy RC (2014) International Conference on Harmonisation. Encyclopedia of Toxicology (3rd Edition) pp. 1070-1072.
- Wagner BA, Buettner GR (2023) Stability of aqueous solutions of ascorbate for basic research and for intravenous administration. Adv Redox Res 9: 100077.
- Sheraz M, Ahmed S, Shahnavi I, Vaid F, Iqbal K (2011) Formulation and Stability of Ascorbic Acid in Topical Preparations. Syst Rev Pharma 2: 86-90.
- Matharoo NS, Garimella HT, German C, Przekwas AJ, Michniak-Kohn B (2023) A Comparative Evaluation of Desoximetasone Cream and Ointment Formulations Using Experiments and In Silico Modeling. Int J Mol Sci 24(20): 15118.
- Olejnik A, Goscianska J, Zielinska A, Nowak I (2015) Stability determination of the formulations containing hyaluronic acid. Int J Cosmet Sci 37(4): 401-407.
- Milosheska D, Roškar R (2022) Use of Retinoids in Topical Antiaging Treatments: A Focused Review of Clinical Evidence for Conventional and Nanoformulations. Adv Ther 39(12): 5351-537.
- Mukherjee S, Abhijit D, Vandana P, Hans CK, Alexander R, et al. (2006) Retinoids in the treatment of skin aging: an overview of clinical efficacy and safety. Clin Interv Aging 1(4): 327-348.
- Cordero-Ramos J, Vicente MB, Mercedes DV, Rubén Barros-T, Manuel Cameán-F, et al. (2022) Formulation, long-term physicochemical and microbiological stability of 15% topical resorcinol for hidradenitis suppurativa. Eur J Hospital Pharma 29(6): 313-318.
- Trissel LA, Ashworth LD, Ashworth J (2018) Salicylic Acid - Sodium Phenylbutyrate. in Trissel’s Stability of Compounded Formulations (6th edition).
- Bhalerao SS, Harshal AR (2003) Preparation, optimization, characterization, and stability studies of salicylic acid liposomes. Drug Dev Ind Pharm 29(4): 451-467.
- Panyachariwat N, Steckel H (2014) Stability of urea in solution and pharmaceutical preparations. J Cosmet Sci 65(3): 187-95.






























