Evaluation of the Filling Effects and Safety of an Injectable Gel Based on A Multi-Crosslinked Hyaluronan (Mu.C.H) Technology for the Correction of Facial Ageing Defects
Ezio Costa*
Clinica Ezio Costa, Sona, Verona, Italy
Submission: March 25, 2024;Published: April 03, 2024
*Corresponding author: Ezio Costa, Clinica Ezio Costa, Via Friuli 5, 37060 Sona, Verona, Italy, Email: ezio@dottoreziocosta.it
How to cite this article: Ezio Costa*. Evaluation of the Filling Effects and Safety of an Injectable Gel Based on A Multi-Crosslinked Hyaluronan (Mu.C.H) Technology for the Correction of Facial Ageing Defects. JOJ Dermatol & Cosmet. 2024; 5(5): 555678. DOI: 10.19080/JOJDC.2024.05.555678
Abstract
Background: Hyaluronic acid (HA) plays a crucial role in skin health: it moisturizes, supports connective tissue and supports collagen production. With increasing age, HA levels decrease leading to wrinkles and a loss of facial volume. Hyal System DUO, an HA-based filler, addresses these defects through a unique combination of auto-crosslinked HA and BDDE-crosslinked HA. Purpose: To evaluate the safety, tolerability, and long-term performance of the HA-based filler in correcting facial wrinkles and restoring volume, turgor and elasticity to the skin.
Materials and Methods: In a single-center observational study, 27 subjects were examined who were treated with the HA-based dermal filler in various areas of the face. Performance was assessed at six visits using the Wrinkle Severity Rating Scale (WSRS), the Facial Volume Loss Scale (FVLS), and the Global Aesthetic Improvement Scale (GAIS). Safety was monitored by recording adverse events.
Results: The HA-based filler showed significant and sustained performance in reducing wrinkles and restoring facial volume over the entire study period. Subject satisfaction was consistently high, with self-reported improvements consistent with physician’s assessments. Adverse events were mild and transient, demonstrating the safety of the product. The cross-linking technologies in this HA-based filler contributed to its lasting effect, providing a natural improvement for facial rejuvenation.
Conclusion: This study demonstrates the safety, tolerability, and performance of Hyal System DUO in the treatment of facial wrinkles and the restoration of volume. The unique combination of auto-crosslinked polymer and BDDE-crosslinked HA showed a progressive and lasting effect on the skin’s appearance. No serious adverse events were reported, underlining the safety profile of the product.
Keywords: Hyaluronic acid; Facial rejuvenation; Wrinkle reduction; Hyal System DUO; Auto-Crosslinked HA
Introduction
Hyaluronic acid (HA) is an essential structural element of connective tissue consisting of repeating disaccharide units of N-acetylglucosamine and D-glucuronic acid [1-3]. It is synthesized in the plasma membrane of fibroblasts and various cells and plays a fundamental role in the human dermis. In the skin, where more than 50% of the body’s HA is located, it performs several functions, including moisturizing, lubricating, stabilizing connective tissue, and supporting collagen production. As we age, the HA content of the skin decreases, resulting in reduced hydration and elasticity. This leads to the appearance of dynamic and static wrinkles, wrinkles from repetitive muscle movement - such as laugh lines, smile lines and crow’s feet - as well as a global loss of facial volume and gravity-induced laxity [3].
To combat the effects of aging, traditional methods of facial rejuvenation have mainly concentrated on tightening the skin through surgical resection and superficial skin resurfacing [3]. In the last two decades, the focus has increasingly shifted to non-incisional, minimally invasive procedures such as the use of facial fillers; these gels replace the hydrodynamic volume within the extracellular matrix, effectively augmenting the soft tissue and reducing the clinical manifestations of the aging process [3,4]. Since 1893 various injectable products have been used to restore facial volume, e.g. autologous fat, liquid paraffin, silicone oil, polytetrafluoroethylene, and bovine collagen. However, these treatments were often associated with serious complications, including inflammatory reactions, that occasionally led to ulcers, fistulas and necrosis. In 2003, the FDA approved HA-based dermal fillers, which have since become the most used fillers. The natural occurrence of HA in the skin, the low risk of side effects and the ability to bind large amounts of water, which help to moisturize the skin and improve skin turgor, made HA the preferred choice for dermal fillers [5,6]. Several HA-based dermal fillers are currently used for facial rejuvenation, which differ in the length of the polymer chain, HA concentration, particle size, gel consistency, gel hardness, gel viscosity, degree of water solubility and degree of cross-linking [7]. The crosslinking process, in which a crosslinking agent binds the HA polymer chains and converts the viscous liquid into a gel, is crucial to prevent enzymatic and free radical degradation of HA dermal fillers [5,7]. Non-crosslinked HA polymers have a half-life of 1 to 2 days in tissue and are not able to maintain the persistence required for a dermal filler; therefore, crosslinking is an essential step to maintain the effect of the injected filler. Currently, two crosslinking agents are used for HA dermal fillers: 1,4-butanediol diglycidal ether (BDDE) and di-vinyl sulfone (DVS). Both substances provide similar results in delaying enzymatic and free radical degradation, but BDDE is the most used substance due to its stability, biodegradability, and proven safety over time [8]. In the last decade, an HA-based filler (Hyal System DUO; Fidia Farmaceutici S.p.A., Abano Terme, Italy) has been introduced to the market. This product, with an HA concentration of 2.5%, is based on Multi-Crosslinked Hyaluronan (Mu.C.H) Technology, in particular contains HA Auto-Crosslinked Polymer (ACP) [9] and HA Crosslinked with BDDE [10]. This combination leads to a long-term correction of skin defects thus providing a durable filling effect, improving skin firmness and elasticity.
The aim of this study was to investigate the safety and longterm performance of this HA-based filler in correcting facial wrinkles and restoring volume, firmness, and elasticity of skin.
Materials and Methods
Study Design
This single-center observational study was conducted at the Ezio Costa Clinic (Sona, Verona, Italy) to evaluate the long-term performance and safety of Hyal System DUO in treating moderate to deep wrinkles and restoring volume and elasticity in various areas of the face, including the forehead, malar region, nasolabial, upper, and lower perioral area, and upper and lower lip regions. Data from 27 subjects treated with the study product were analyzed. All participants provided signed informed consent and attended six appointments over the course of the study (T0 to T5). At baseline (T0), various subject data were collected prior to treatment, including demographic data, skin type according to the Fitzpatrick classification [11], degree of photoaging according to the Glogau classification [12], and information on concomitant diseases and concomitant or recent pharmacologic treatments within the last 30 days. In addition, subjects had to provide information on skin treatments they had received in the last 12 months for corrective aesthetic purposes, previous procedures with permanent fillers, their tendency to develop hypertrophic, atrophic or cheloid scars, and local skin diseases on the face such as infections, dermatitis, psoriasis, eczema, or herpes.
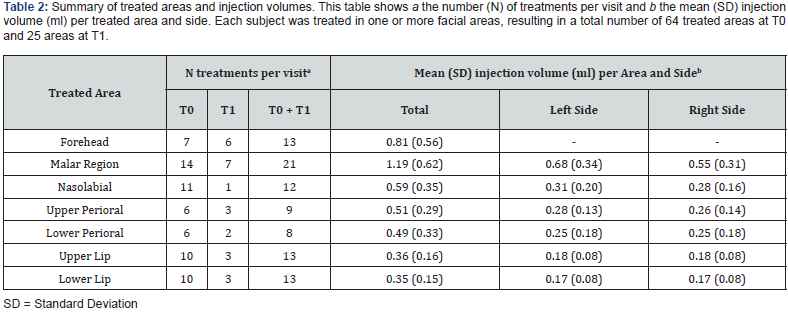
After collecting this data, subjects were treated with the HAbased dermal filler in one or more regions, which comprised the forehead, malar region, nasolabial, upper, and lower perioral area, and upper and lower lip regions, resulting in a total of 64 treatments administered across these specified areas.
The first follow-up examination (T1) took place two weeks after the T0 treatment. All subjects were examined at T1 for followup. In addition, few specific regions that required a touch up were retreated at this visit (T1), for a total of 25 areas in 13 subjects. Follow-up examinations were then carried out at T2, T3, T4 and T5 at 1, 3, 6 and 9 months respectively after the T0 treatment.
The condition of the face was assessed using various scales. In particular, the Wrinkle Severity Rating Scale (WSRS) [13] was used to evaluate the facial wrinkles; despite this scale was developed for the nasolabial folds, we extended its application to all types of wrinkles. The loss of facial volume was evaluated using the Facial Volume Loss Scale (FVLS) [14]. Both scales include five levels from 1 to 5, with 5 being the most severe, and rated by a physician [13,14]. In addition, subjects rated their aesthetic improvement at all visits except T0 using a Global Aesthetic Improvement Scale (GAIS) [15,16]. The scale ranges from 1 to 5, with a score of 5 representing “worse”, a score of 4 representing “no change”, a score of 3 representing “improved”, a score of 2 representing “much improved” and a score of 1 representing “very much improved” [16].
For the analysis, all 5-point scales - WSRS, FVLS, and GAIS - were divided into 3 rating categories (i.e. “1-2”, “3”, and “4-5”). Finally, subjects completed a satisfaction questionnaire to provide an overall assessment of the treatment, including the procedure and outcome. The questionnaire included a numerical rating scale (NRS) ranging from 1 to 10, with 1 indicating no satisfaction and 10 indicating complete satisfaction.
Photographs of subjects’ faces were taken at each time point using the Vectra 3D imaging system and digital reflex camera (in 5 positions: front, 45° right and left and right and left profile). Subjects were monitored up to 20 minutes after the injection to identify immediate reactions, and adverse events (AEs) were consistently monitored throughout the entire study period.
Population
The study included twenty-seven participants who met the inclusion criteria, i.e. were at least 18 years old, had undergone treatment with the Hyal System DUO in areas such as the forehead, malar region, nasolabial area, perioral area, and lips, and provided signed informed consent. Exclusion criteria included subjects who had received any skin treatments for cosmetic correction, such as biomaterial implants, lifting, botulinum toxin injections, laser, or chemical peels, within the 12 months prior to starting treatment with the study product.
Objectives and Endpoints
The primary objective was to assess the sustained performance of treatment 6 months (T4) after the first treatment (T0), with the primary endpoint focused on the comparison of subject proportions in the three defined WSRS categories between T4 and T0. The secondary objective was to investigate the performance of the treatment over time. In this regard, the endpoints of the study included i) the assessment of the distribution of subjects in the three predefined WSRS categories at T2, T3, T4 and T5 compared to the distribution of WSRS categories at T0; ii) to compare the distribution of subjects in the FVLS categories at T2, T3, T4 and T5 with the distribution of categories at baseline (T0); iii) to examine the distribution of subjects in the GAIS categories between T5 and T1; iv) to analyze the level of subject satisfaction during the study period. The safety endpoint included the recording of the occurrence and type of adverse events during the entire study period.
Injection Procedure
Hyal System DUO consists of a sterile, non-pyrogenic, clear and transparent gel obtained by combining HA crosslinked with BDDE and ACP HA. It is supplied in a pre-filled disposable syringe consisting of a 2.5% HA concentration, sodium chloride and water for injectable preparations. The product was injected into the deep dermis or hypodermis using a 27 or 30 G needle or 25 G cannula depending on treating procedures. In particular, injections were administered into the deep dermis for the forehead and nasolabial areas and into the hypodermis for the malar region, perioral, and lip areas. Various injection techniques were available to the physician, including retrograde linear threading, bolus, and serial puncture techniques. The procedure was performed under lidocaine topical anesthesia if the patient desired. After injection of the product, the treated area was gently massaged to distribute the product evenly throughout the tissue.
Statistical Analysis
Subject characteristics were summarized and tabulated by time, either as counts and percentages or as mean and standard deviation (SD) for categorical and continuous variables, respectively. The 5-point scales WSRS, FVLS and GAIS were categorized into 1-2, 3 and 4-5 points. Response variables were analyzed using a generalized ordinal model with repeated measures for multinomial data, with a cumulative logit link function and an independent correlation working matrix to model the correlation of subjects’ responses. Visit time was included as a fixed factor in the model. Pairwise comparisons of changes in scores for the response variables between follow-up and baseline were tested for significance using the z-test. All tests were twotailed tests and were considered significant at the 5% level. The significance levels for the main endpoints were adjusted for multiple comparisons using the Tukey-Kramer method. All analyzes were performed with SAS 9.4 (NC, Cary).
Results
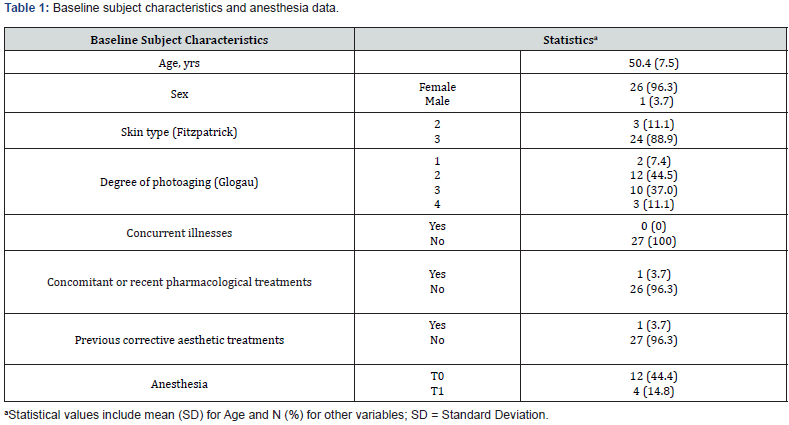
The study included 27 participants who met the inclusion criteria (26 women (96.3%) and one man). At baseline, the mean (SD) age was 50.4 (7.5) years and ranged from 37 to 63 years. The majority of participants (88.9%, N = 24) had a Fitzpatrick phenotype of 3, while just over 80% had a photoaging degree between 2 and 3. Only one subject was receiving concurrent pharmacologic treatment for thyroid dysfunction. During the injection procedure, 12 subjects received local anesthesia at T0, while only 4 subjects received local anesthesia at T1. All relevant data are listed in Table 1. A total of 64 areas were treated at T0, and 25 areas were touched up at T1, resulting in a total of 89 treatments. Injection volumes ranged from 0.1 ml to 2 ml, with the mean injection volume varying across different areas and sides, as illustrated in Table 2. With regard to the primary endpoint, a statistically significant change (P = 0.003) was observed in the distribution of subjects across the three predefined categories (1-2, 3 and 4-5) of the WSRS scale between T4 and T0 [Figure 1 (red arrow), Table 3]. As shown in Table 3, none of the subjects were categorized in the mild severity category (category 1-2) at baseline; however, after six months there was a significant shift: 16 subjects (59.3%) fell into this category, and the number of subjects in category 4-5 decreased from 26 (96.3%) to zero. The remaining subjects (N = 11; 40.7 %) fell into category 3 (moderate).
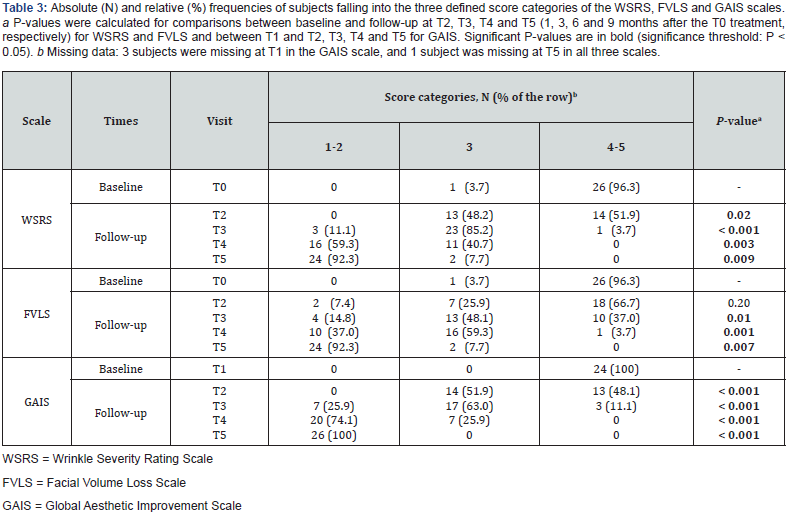
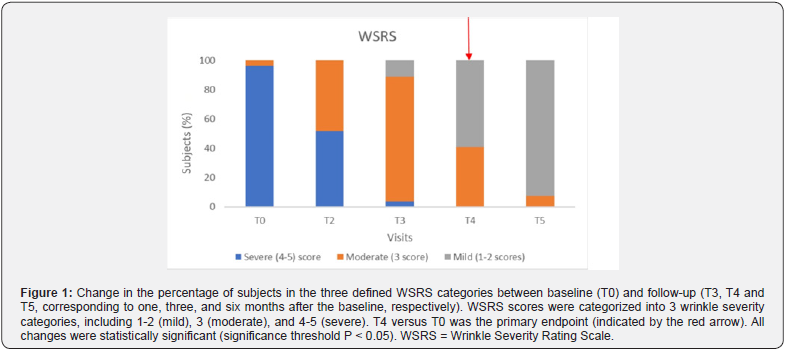
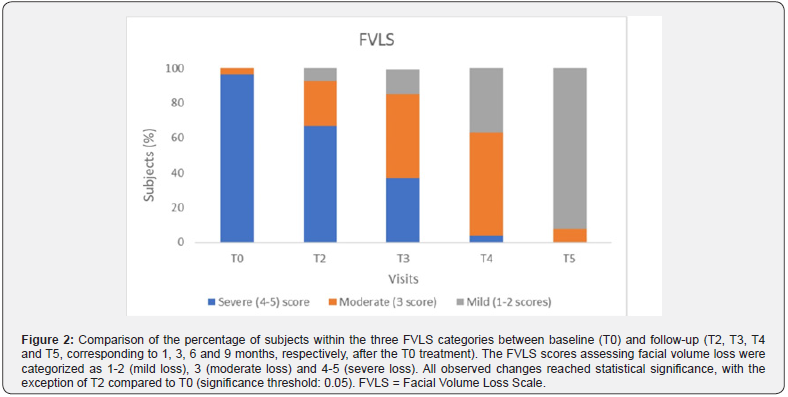
The effectiveness of the treatment was demonstrated by examining secondary endpoints that assessed its performance over time. As shown in Figure 1 and detailed in Table 3, a significant reduction in wrinkles was observed over time, as evidenced by the evolving distribution of subjects across the three WSRS categories. Subjects in category 1-2 progressed from zero at baseline to 24 (92.3%) nine months post-treatment (T5), with intermediate values at T2, T3 and T4 (0%, 11.1% and 59.3%, respectively), indicating progressive and sustained performance of the product. A parallel trend was seen in the distribution of subjects across the three score categories of the FVLS scale, as shown in Figure 2 and summarized in Table 3. The proportion of subjects in category 1-2 increased significantly (from 0% at T0 to 92.3% at T5), while there was a significant decrease in category 4-5 (from 96.3% at T0 to 0% at T5), with values at intermediate time points in between.
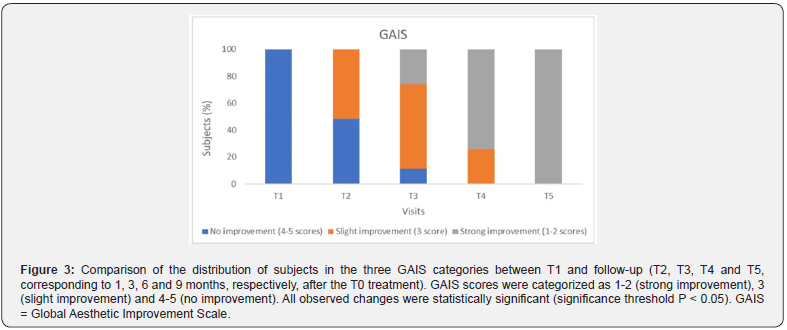
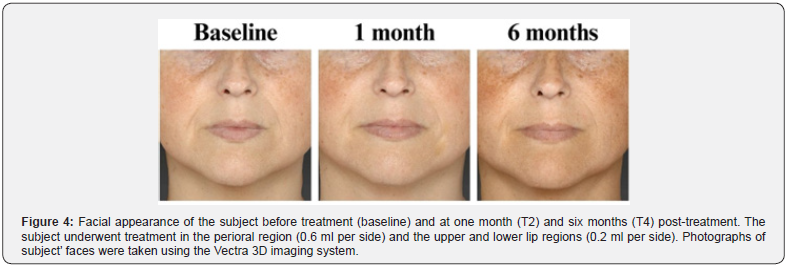
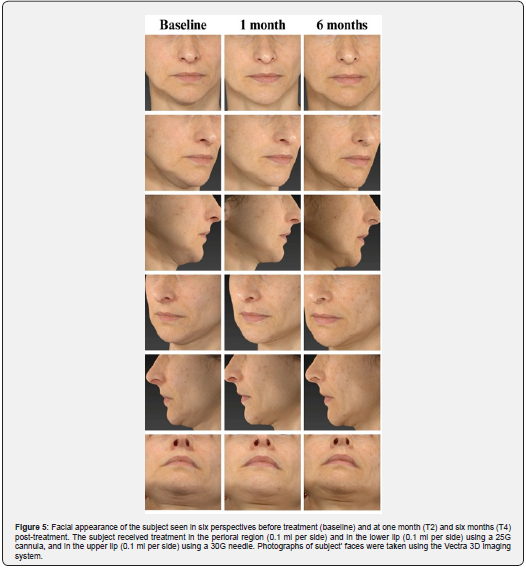
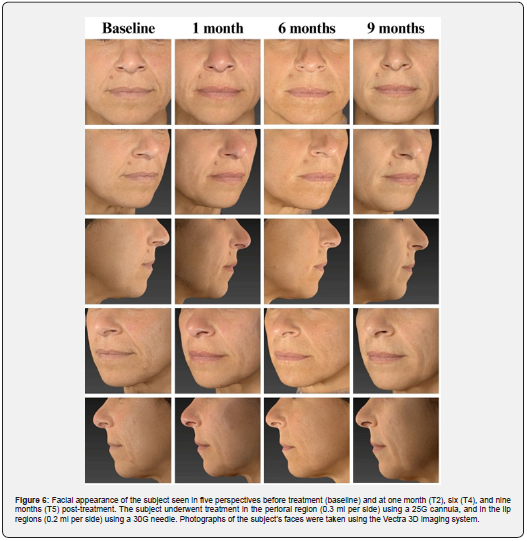
These results were further confirmed by the GAIS evaluation shown in Figure 3 and summarized in Table 3. The shift in subject distribution from category 4-5 to category 1-2 was evident, with all subjects falling into category 4-5 at T1 and moving into category 1-2 nine months later (T5). This shift underlines a significant improvement as perceived by the subjects themselves. The results of the satisfaction questionnaire are consistent with these findings and showed consistently high mean scores of between 8.9 and 9 (out of 10) throughout the study period (data not shown). Figure 4 and 5 shows the outcomes of the HA-based dermal filler after one- and six-months post-treatment, and Figure 6 shows the results up to nine months post-treatment.
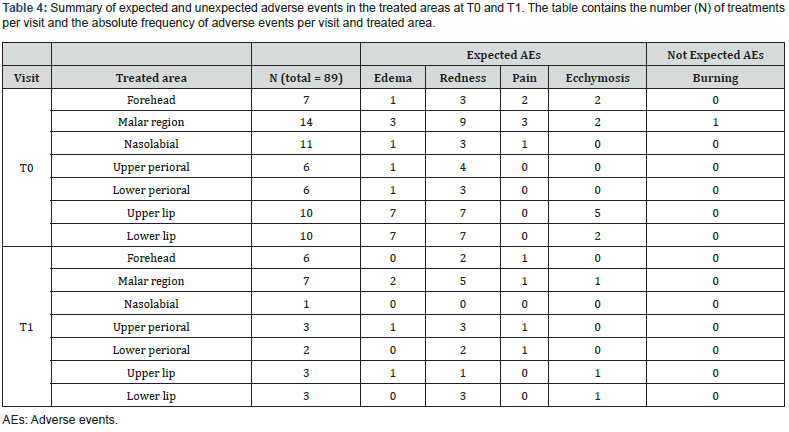
There were no reports of serious adverse events during the entire duration of the study. After treatment, local reactions at the injection site were observed in some treated areas. These reactions, which were expected and not severe, were directly related to the treatment procedure. The most common reaction was redness, which occurred in 58.4% of the treated sites. Mild edema and pain were noted in 28.1% and 10% of injected areas, respectively. The redness disappeared on the same day of treatment, while edema and pain subsided within at least 15 minutes and up to a maximum of 3 days. In addition, a few cases of ecchymosis were reported (15.7%), but these were due to the procedure rather than the treatment itself and resolved in a few days. In addition, only one subject reported a burning sensation in the malar region, which resolved within 5 minutes. All data are shown in Table 4.
Discussion
The crosslinking process enables the HA used in dermal fillers to provide a durable filling effect [4]. Two different crosslinking technologies are used in the HA-based filler examined in this study: HA Auto-Crosslinked Polymer (ACP) [9] and HA Crosslinked with BDDE [10]. The combination of these technologies ensures the long-term performance of the filler and protects it from enzymatic and free radical degradation [5]. The present study confirms this durability and shows a progressive effect of the Hyal System DUO on wrinkle reduction and improvement of skin firmness over time (Figure 4, 5, and 6). The most pronounced performance was seen nine months after initial treatment, with 92.3% of subjects scoring in the 1-2 category on both the WSRS and FVLS scales. Interestingly, the positive effects were evident earlier, two weeks after treatment for wrinkle reduction and one month after treatment for volume restoration. This result indicates a gradual release of HA that support a consistent and lasting filling effect on the skin.
In addition to the assessments based on the changes in WSRS and FVLS values, participants reported sustained aesthetic improvements, as evidenced by their GAIS scores: nine months post-treatment, all subjects achieved the highest rating of “very much improved” on the GAIS scale, reflecting a progressive global aesthetic enhancement from the patients’ viewpoint. A similar effect was observed by Sparavigna et al. in a prospective study of 20 subjects treated with the same HA-based filler for nasolabial folds. The study showed the most significant reduction in wrinkles after 6 months, with the treatment effect persisting in more than half of the subjects after 9 months [17]. In addition, a significant improvement in facial volume was observed from the first weeks to 12 months after treatment [17]. Further studies by Fino and colleagues confirmed the performance of the product in the correction of nasolabial folds, showing visible effects after 3 and 6 months compared to baseline [18].
This study demonstrated not only the performance of the study product, but also its safety and tolerability. Adverse events were expected local reactions following treatment, including redness (58.4%), mild edema (28.1%), pain (10%), and ecchymoses (15.7%). The ecchymoses were related to the procedure, resulting from the extravasation of blood following perforation of the skin vessels by the needle. All reported symptoms, consistent with those documented in the literature [17-19], naturally resolved within hours or, at most, a few days. No hypersensitivity reactions or inflammation were reported, indicating a safe profile of the product. Hyaluronic acid is a natural biological component of tissue and is non-toxic and non-immunogenic. However, it is known that the crosslinking process can produce unreacted or residual crosslinking agents in the final product, which are potentially toxic at elevated concentrations if they are not bound to other molecules. The BDDE crosslinking agent used in Hyal System DUO is neutralized by water or hydroxide, so that only minimal amounts of unreacted BDDE are present in the final product. These traces are well below the threshold above which there is a health risk to humans. This study has some limitations, including a limited sample size and a lack of control group. Conducting further research with a larger sample size and implementing a comparative group could provide additional support for these findings.
Summary
In summary, this study provides evidence for the safety, tolerability, and performance of the HA-based dermal filler Hyal System DUO. It shows a sustained reduction in wrinkles, restoration of facial volume, and improvement in facial appearance, with no serious adverse events reported by subjects.
References
- Kim JE, Sykes J (2011) Hyaluronic Acid Fillers: History and Overview. Facial Plast Surg 27(06): 523-528.
- Manuskiatti W, Maibach HI (1996) Hyaluronic Acid and Skin: Wound Healing and Aging. Int J Dermatology 35(8): 539-544.
- Buck DW, Alam M, Kim JYS (2009) Injectable fillers for facial rejuvenation: A review. J Plast Reconstr Aesthet Surg 62(1): 11-18.
- Guarise C, Barbera C, Pavan M, Panfilo S, Beninatto R, et al (2019) HA-based dermal filler: downstream process comparison, impurity quantitation by validated HPLC-MS analysis, and in vivo residence time study. Journal of Applied Biomaterials & Functional Materials 17(3): 228080001986707.
- Tezel A, Fredrickson GH (2008) The science of hyaluronic acid dermal fillers. Journal of Cosmetic and Laser Therapy 10(1): 35-42.
- Krauss MC (1999) Recent advances in soft tissue augmentation. Seminars in Cutaneous Medicine and Surgery. 18(2): 119-128.
- Mansouri Y, Goldenberg G (2015) Update on Hyaluronic Acid Fillers for Facial Rejuvenation. Cutis 96(2): 85-88.
- De Boulle K, Glogau R, Kono T, Nathan M, Tezel A, et al (2013) A Review of the Metabolism of 1,4-Butanediol Diglycidyl Ether-Crosslinked Hyaluronic Acid Dermal Fillers. Dermatologic Surgery 39(12): 1758-1766.
- Pluda S, Pavan M, Galesso D, Guarise C (2016) Hyaluronic acid auto-crosslinked polymer (ACP): Reaction monitoring, process investigation and hyaluronidase stability. Carbohydr Res 433: 47-53.
- Guarise C, Pavan M, Pirrone L, Renier D (2012) SEC determination of cross-link efficiency in hyaluronan fillers. Carbohydrate Polymers 88(2): 428-434.
- Fitzpatrick TB (1988) The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol 124(6): 869-871.
- Glogau RG (1996) Aesthetic and anatomic analysis of aging skin. Seminars in Cutaneous Medicine and Surgery 15(3): 134-138.
- Day DJ, Littler CM, Swift RW, Gottlieb S (2004) The Wrinkle Severity Rating Scale: A Validation Study. American Journal of Clinical Dermatology 5(1): 49-52.
- Ascher B, Coleman S, Alster T, Bauer U, Burgess C, et al Full Scope of Effect of Facial Lipoatrophy: A Framework of Disease Understanding. Dermatol Surg 32(8): 1058-1069.
- Narins RS, Brandt F, Leyden J, Lorenc ZP, Rubin M, et al (2003) A Randomized, Double-Blind, Multicenter Comparison of the Efficacy and Tolerability of Restylane Versus Zyplast for the Correction of Nasolabial Folds. Dermatol Surg 29(6): 588-595.
- Alessandrini A, Fino P, Giordan N, Amorosi V, Scuderi N (2015) Evaluation of a new hyaluronic acid dermal filler for volume restoration. J Cosmet Laser Ther 17(6): 335-342.
- Sparavigna A, Fino P, Tenconi B, Giordan N, Amorosi V, et al (2014) A new dermal filler made of cross‐linked and auto‐cross‐linked hyaluronic acid in the correction of facial aging defects. J of Cosmetic Dermatology 13(4): 307-314.
- Fino P, Toscani M, Grippaudo FR, Giordan N, Scuderi N (2019) Randomized Double-Blind Controlled Study on the Safety and Efficacy of a Novel Injectable Cross-linked Hyaluronic Gel for the Correction of Moderate-to-Severe Nasolabial Wrinkles. Aesth Plast Surg 43(2): 470-479.
- Chiang YZ, Pierone G, Al‐Niaimi F (2017) Dermal fillers: Pathophysiology, Prevention, and Treatment of Complications. Acad Dermatol Venereol 31(3): 405-413.






























