The Rejuvenating Effect and Tolerability of an Auto-Crosslinked Hyaluronic Acid on facial and neck wrinkles
Ezio Costa*
Clinica Ezio Costa, Sona, Verona, Italy
Submission: March 25, 2024;Published: April 03, 2024
*Corresponding author: Ezio Costa, Clinica Ezio Costa, Via Friuli 5, 37060 Sona, Verona, Italy, Email: ezio@dottoreziocosta.it
How to cite this article: Ezio Costa*. Adipose Derived-Stem Cells and their Cell-Free Supernatant Combined with a Nanofiber Scaffold for Skin Rejuvenation: The Avancell Stem Cell SecretomeTM - A Technical Note. JOJ Dermatol & Cosmet. 2024; 5(5): 555677. DOI: 10.19080/JOJDC.2024.05.555677
Abstract
Introduction: Aging of the face is associated with loss of volume, wrinkles, and laxity. Injectable formulations particularly those based on hyaluronic acid (HA), are a popular non-surgical option. Hyal System ACP, an HA-based injectable formulation with Auto-Crosslinked Polymer (ACP) Technology, aims to give a rejuvenating effect to the skin by correcting soft tissue defects. In this study, performance and safety of the Auto-Crosslinked HA have been investigated on facial and neck wrinkles.
Materials and Methods: In a monocentric, open-label study, 22 subjects treated with Auto-Crosslinked HA in the periocular, perioral and neck areas were studied. Validated scales were used to measure wrinkles (WSRS) and aesthetic improvement (GAIS) at baseline and at one, three and six months after treatment. Photographs of the subjects’ faces were taken at the same time points using Vectra 3D imaging systems. Subject satisfaction and adverse events were recorded throughout the study.
Results: A significant reduction in high-severity wrinkles (WSRS) was observed at one, three and six months, with a greater effect at six months. Similar trends were observed for aesthetic improvement (GAIS), with positive responses increasing over time. Subject satisfaction reached 8.8 out of 10 points after six months. In terms of safety, the expected local events such as redness and swelling were frequent but transient, whereas ecchymoses (5.9%) were more persistent, resolving in a few days. No serious adverse events were reported.
Conclusions: The study demonstrated the safety and long-lasting performance of Auto-Crosslinked HA in reducing wrinkles and rejuvenating the skin. Participants indicated high satisfaction and perceived improvement according to the GAIS scale.
Keywords: Hyal System ACP; Auto-Crosslinked HA; Hyaluronic acid; Facial rejuvenation; Wrinkle reduction
Introduction
With age, facial skin changes due to loss of facial volume, the appearance of wrinkles caused by repetitive movements of the facial muscles and sagging of the skin due to gravity [1]. Several factors contribute to these changes, such as sun exposure, smoking habits, menopause, resorption of bony structures, redistribution of subcutaneous fat, and skin damage [2,3]; however, primarily, these changes are attributed to a decline in hyaluronic acid (HA) levels in the skin [4]. To achieve facial rejuvenation, non-surgical methods such as soft tissue augmentation with facial injectable formulations have become increasingly popular in recent years [1,3]. There are currently a few of several types of injectable formulations available such as autologous fat, collagens, biosynthetic polymers, and HA [1,4]. All these injectable formulations are used to replenish the hydrodynamic volume of the extracellular matrix to mitigate the effects of skin aging [5]. Due to its biocompatible, reversible, and hygroscopic properties, HA is the most used injectable substance for skin rejuvenation [4,5]. It is a high molecular weight polysaccharide synthesized in the plasma membrane of fibroblasts and other cells [6]. The HA in the skin makes up over 50% of the total HA in the body, but is degraded by sunlight and aging [6,7]. HA-based injectable formulations are designed to rejuvenate the skin by replenishing HA and retaining water, which in turn minimizes skin sagging and wrinkles [7]; therefore, these formulations are typically used to treat wrinkles, fill in folds, and volumize specific regions [4]. As HA is a polymer with high water solubility that is rapidly degraded by natural skin enzymes and free radicals, chemical modification with crosslinkers is required to develop HA-based injectable formulations [8]. The crosslinkers bind HA polymer chains together, transforming the viscous liquid into a gel that provides physical and chemical protection against degradation by enzymes and free radicals [8]. This results in less degradation of the chains and ultimately a longer retention time of HA in the skin, from 6 to 18 months [5,8]. Several HA-based injectable formulations are currently available on the market, which differ in terms of degree of cross-linking, polymer chain length, HA concentration, particle size, gel consistency, gel hardness, gel viscosity, and water solubility [9]. Hyal System ACP is an HA-based injectable formulation manufactured by Fidia farmaceutici S.p.A. (Abano Terme, Padua, Italy). It is a hydrogel containing a patented low molecular weight HA that has been subjected to an auto-crosslinking reaction, based on the proprietary ACP (Auto-Crosslinked Polymer) Technology, and characterized by a gradual release of HA after injection [10]. The Auto-Crosslinking Technology is based on inter- and intra-molecular ester bonds between HA polymers that does not contain residual agents, thus ensuring a high safety profile [10]. The purpose of the ACP HA is to correct minor soft tissue defects, improve and maintain the turgor and the elasticity of the skin, and thus achieve a rejuvenating effect on the face [11]. The aim of the present study is to collect data on performance and safety of the ACP HA injectable formulation on facial and neck wrinkles.
Materials and Methods
Population
This open-label, monocentric, observational study was conducted at the Ezio Costa Clinic, Sona, Verona, Italy, to evaluate the performance and safety of ACP HA in rejuvenating the periocular, perioral and neck areas over time. The analysis included data from subjects aged 18 years and older who signed informed consent and received ACP HA treatment in the periocular and/or perioral and/or neck region. Data from subjects who had undergone skin treatments for aesthetic correction, such as biomaterial implants, lifting, botulinum toxin injections, laser, or chemical peels within the 12 months prior to starting treatment with the ACP HA were excluded. All participants agreed to participate in the study and provided written informed consent.
Study Design
The analysis was performed using data collected at six different time points (from T0 to T5). At the first three appointments (T0, T1 and T2), which took place every two weeks, one or more facial regions were treated, adopting personalized treatment protocols to fit consumer requirements; at the T1 and T2 appointments also a follow-up examination was carried out - before the injection was repeated - in order to check the outcome of the previous injections. The last three visits (T3, T4, and T5) were planned as follow-up, respectively one, three, and six months after the baseline (T0). At the beginning of the study (T0), a range of pre-treatment information was collected, including demographic data, skin type according to the Fitzpatrick classification [12] and the degree of photoaging according to the Glogau classification [13]. In addition, data was collected on the subject’s general health, including any concomitant diseases, concomitant or recent pharmacological treatments within the last 30 days, any skin treatments for corrective aesthetic purposes in the last 12 months, previous procedures with permanent fillers, tendency to develop hypertrophic, atrophic or cheloid scars, and any local skin pathologies on the face (such as infections, dermatitis, psoriasis, eczema, and herpes). Photographs of the subjects’ faces were taken at baseline and at the T3, T4 and T5 follow-up visits using Vectra 3D imaging systems.
Subjects were observed up to 20 minutes post-injection to detect immediate reactions, while adverse events (AEs) were continuously monitored throughout the study duration.
Data Collected and Scale Scores
The severity of wrinkles was assessed at each time point by the physician through the appropriate Wrinkle Severity Rating Scale (WSRS) [14], using photographs and verbal descriptions; despite this scale was developed for the nasolabial folds, we extended its application to all types of wrinkles. The scale comprises grades 1 to 5, with grade 5 being the most severe. Grade 1 is characterized by the absence of folds and a continuous skin line. Grade 2 is defined as mild, with shallow but visible folds and a slight indentation. Grade 3 denotes a medium grade with moderately deep folds and a pronounced facial shape that is visible with normal appearance but not with stretching. Grade 4 is classified as severe, with very long and deep folds, pronounced facial features and < 2 mm visible folds when stretched. Grade 5 is characterized by extremely deep and long folds that affects the appearance of the face and V-shaped wrinkles of 2-4 mm that are visible when stretched [14].
Feedback from subjects treated was collected at each time point using a satisfaction questionnaire on a numerical rating scale (NRS) from 1 to 10 (where 1 is “not at all satisfied” and 10 is “completely satisfied”). In addition, at all-time points except T0, subjects were given a validated scale, the Global Aesthetic Improvement Scale (GAIS) [15,16], to assess their aesthetic improvement compared to the baseline photo. The GAIS [15,16], scale assesses aesthetic improvement by comparing a live face after treatment with a baseline photo and rating the improvement. The scale ranges from 1 to 5, with 5 being “worse”, 4 “no change”, 3 “improved”, 2 “much improved” and 1 “very much improved”.
For the analysis, the scale scores of WSRS and GAIS were divided into three categories (i.e., 1-2, 3 and 4-5) and the proportion of subjects in each category was used to assess the changes between the different time points.
Objectives and Endpoints
The main objective of the study was to evaluate the longlasting performance of ACP HA in reducing facial wrinkles three months (T4) after the first treatment at baseline (T0). Accordingly, the primary endpoint was to compare the proportion of subjects in the different WSRS score categories between T4 and T0.
The secondary objectives and endpoints were: i) to evaluate the performance of ACP HA on facial wrinkles reduction by comparing the proportions of subjects in the WSRS score categories measured at one (T3), three (T4) and six (T5) months with those at baseline (T0); ii) assessment of subjects’ aesthetic improvement by comparing the proportions of subjects in the GAIS score categories at T3, T4 and T5 with those at T1; iii) assessment of subject satisfaction using the NRS satisfaction questionnaire; iv) assessment of the safety profile of ACP HA by monitoring the rate of expected and unexpected adverse events throughout the study period.
Injection Procedure
Hyal System ACP is a sterile and highly viscous hydrogel obtained by the condensation of hyaluronic acid using the proprietary ACP (Auto-Crosslinked Polymer) Technology [10]. The formulation contains auto-crosslinked hyaluronic acid (2%), sodium chloride and water for injection. The product was administered with a 30 G needle and injected into the superficial dermis using a serial puncture technique. The injection was performed under topical anesthesia (lidocaine) if the subject so desired. After the injection, the treated area was gently massaged to facilitate the distribution of the product in the tissue.
Statistical Analysis
Subject characteristics and subject satisfaction were summarized and tabulated by time, using counts and percentages for categorical variables and means and standard deviations (SD) for continuous variables. The 5-point WSRS and GAIS scales were categorized into 1-2, 3, and 4-5 scores. Response variables were analyzed using a generalized ordinal model with repeated measures for multinomial data, with a cumulative logit link function and an independent correlation working matrix to model the correlation of subjects’ responses. Visit time was included as a fixed factor in the model. Pairwise comparisons of changes in response variable values between follow-up and baseline were tested for significance using the z-test. All tests were two-tailed tests and were considered significant at the 5% level. All analyzes were performed with SAS 9.4 (NC, Cary).
Results
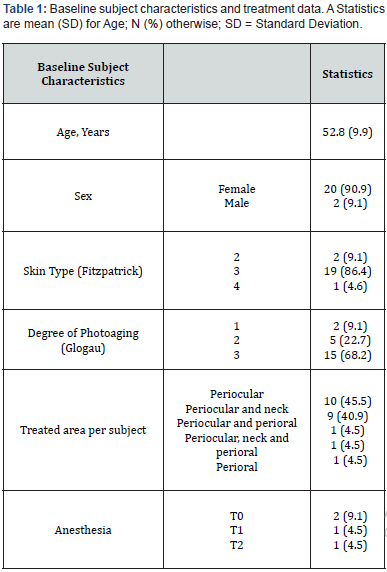
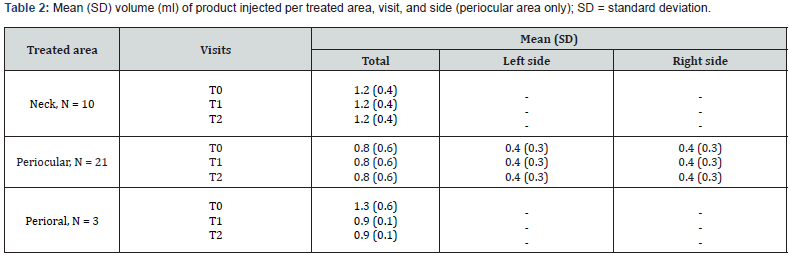
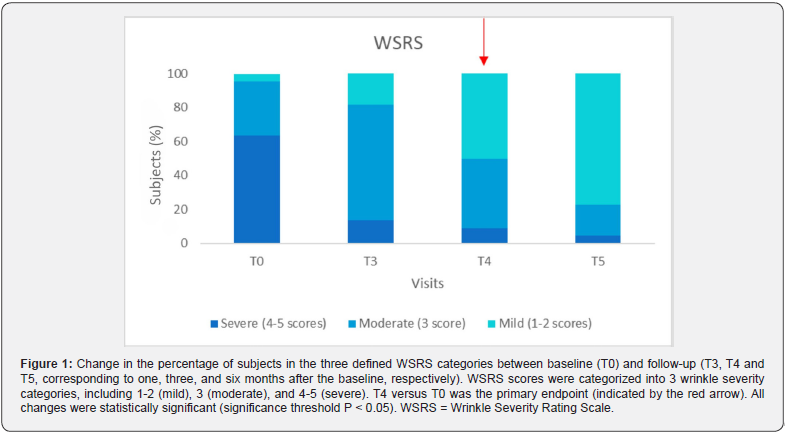
The study included 22 consecutive subjects, with 20 of them being female (90.9%), who received treatment with ACP HA during the initial three appointments (T0, T1, and T2). At baseline, the mean age (SD) was 52.8 (9.9) years and ranged from 29 to 66 years. A significant proportion of participants (86.4%, N = 18) had a Fitzpatrick phenotype of 3, while 68.2% had a photoaging grade of 3 according to the Glogau classification. No subject reported concomitant diseases, nor recent pharmacological or aesthetic treatments. Of the total 22 subjects, 10 were treated only in the periocular area, 9 were treated simultaneously in the periocular and neck areas, 1 was treated in the periocular and perioral areas, 1 was treated simultaneously in all three areas (neck, perioral, and periocular), and 1 was treated only in the perioral area. During the injection procedure, two subjects received topical anesthesia at T0, while only one subject received topical anesthesia at T1 and T2. All data are shown in Table 1. The average injection volume varied between 0.4 and 1.3 ml, depending on the treated area, visit and side of the face, as shown in Table 2. All subjects completed the follow-up examinations. A statistically significant change in the distribution of subjects across the three delineated categories of the WSRS scale between T4 and T0 (P = 0.004) confirms the ACP HA long-lasting effect on wrinkles reduction after 3 months. In particular, the proportion of subjects with highly pronounced wrinkles (WSRS category 4-5) decreased significantly at T4 compared to baseline (from 63.6% to 9.1%). At the same time, the proportion of subjects with WSRS category 1-2 increased significantly compared to baseline (from 4.5% to 50%). Figure 1 and Table 3 provide a detailed and quantifiable explanation for the observed changes. Regarding the secondary endpoints, statistically significant changes were observed in the distribution of subjects across the three defined categories for all scales assessed, particularly between baseline and follow-up at T3, T4 and T5 for WSRS and between T1 and follow-up at T3, T4 and T5 for GAIS, as shown in Table 3.
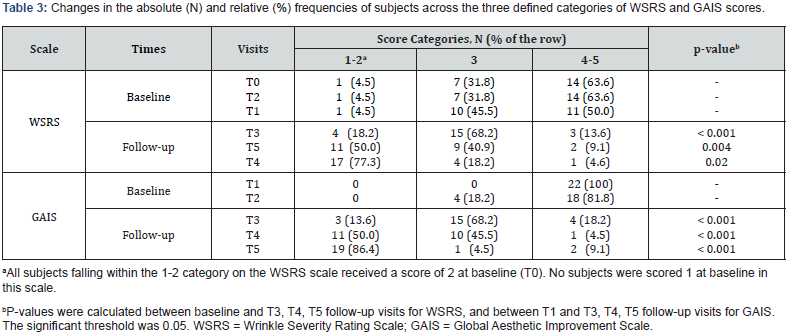
In relation to the WSRS scale, the degree of wrinkle severity was significantly reduced not only at T4, but also at the time points T3 and T5 (Figure 1); indeed, at baseline, only 4.5% of subjects fell into the less severe category (1-2) of WSRS (Table 3) while this proportion increased to 18.2% at T3, to 50.0% at T4 and to 77.3% at T5. Consequently, the number of subjects falling into categories 4-5 decreased significantly.
assessment of their overall aesthetic improvement, confirmed these results. At T1, all subjects were in the least favorable category, but progressed to higher categories over time. At T5, 86.4% of subjects fall into category 1-2, 4.5% in category 3 and 9.1% in category 4-5, indicating a positive trend in perceived aesthetic improvement among subjects (Figure 2; Table 3).
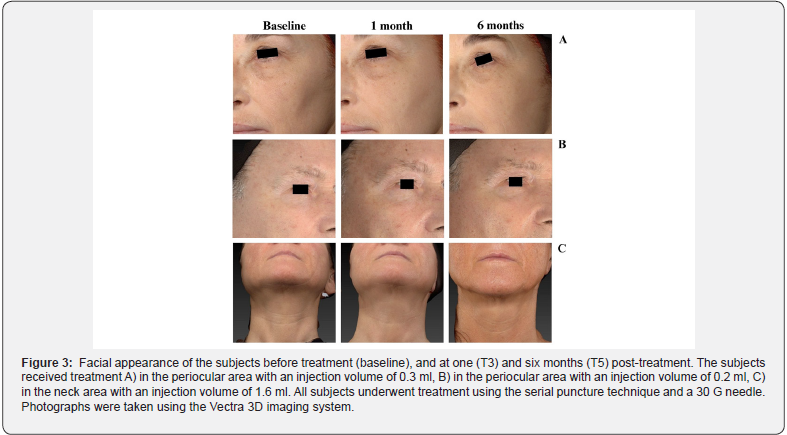
with the procedure and the results. At T0, the satisfaction score was 8.7 ± 0.4 out of 10 and increased to 8.8 ± 0.4 at T5. The subjects’ perception and satisfaction were substantiated by the photographs in Figure 3, illustrating ACP HA results at one- and six-months post-treatment. No serious AEs occurred during the study. As shown in Table 4, most reported AEs were expected localized post-treatment events, such as redness, which resolved within 30 minutes, and mild swelling and ecchymosis, which resolved within a few days.
Discussion
The optimal injectable formulations should be biocompatible, easy to prepare and administer, provide long-lasting cosmetic results, and not cause pain or other complications at the injection site [15]. HA-based injectables best fulfill these requirements. The linear polysaccharide structure of HA, which consists of repeating disaccharide units of N-acetylglucosamine and D-glucuronic acid, results in self-coiling chains that form a viscous and elastic matrix, making them suitable for this purpose [4]. In addition, their high hydrophilicity enables effective moisturization of the skin, which counteracts skin dehydration and wrinkling while improving skin turgor and elasticity. This study provides evidence of the performance of the ACP HA by showing a significant difference in WRSR values between baseline and follow-up visits. The Auto- Crosslinked HA consistently demonstrated its ability to reduce wrinkles and rejuvenate the skin, with visible results one, three and six months after treatment. Of particular note is the maximum performance observed six months after treatment (Figure 3), with 77.3% of subjects achieving WSRS categories of 1-2 (mild). This is in stark contrast to the baseline values (4.5%) and underlines the significant and sustained effect of the ACP HA over time (Figure 1 and Table 3). The prolonged performance of this injectable formulation can be attributed to ACP Technology, a property that protects HA from degradation by hyaluronidase and free radicals; this protective mechanism facilitates a gradual release of HA, capable of spreading homogeneously to tissues, thus restoring of favorable conditions for strengthening the physiological activity of skin, leading to the improvement of aging signs and slow down the aging processes [5,8,10].
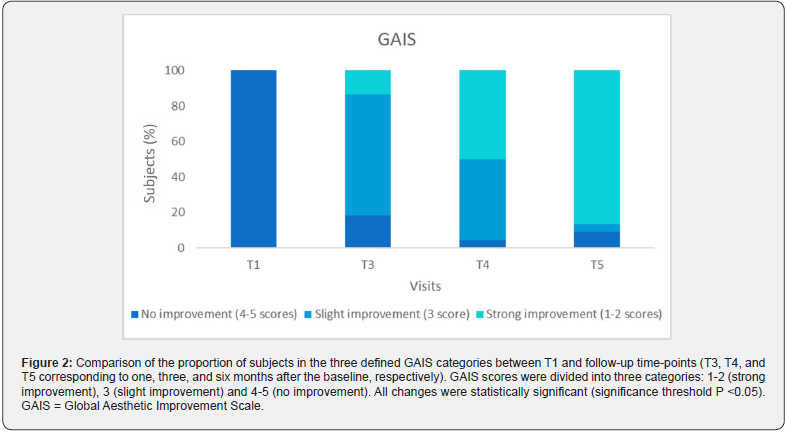
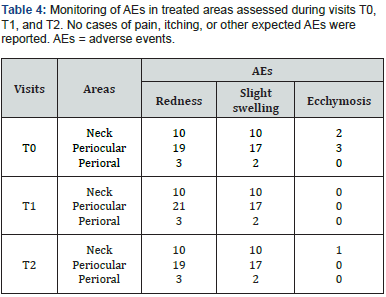
In addition to correcting wrinkles, subjects perceived a remarkable overall improvement in their appearance, which was confirmed by a statistically significant difference in GAIS scores between T1 and the follow-up examinations, providing a rejuvenating effect to the skin. This effect was particularly pronounced at 6 months (T5; see Table 3 and Figure 2), which is consistent with the physician’ objective assessment. Subjects expressed their satisfaction with the effectiveness of the treatment and the treatment experience in a satisfaction questionnaire and achieved an impressive mean score of 8.8 out of 10 on the rating scale at 6 months (T5). Remarkably, none of the subjects experienced pain, inflammation or immunogenic reactions after the injection and no serious adverse events were reported throughout the study. The low immunogenicity of HA-based injectable formulations is a consequence of the natural occurrence of HA as a biological component of the dermis, making it nontoxic to cells and tissues [17]; consequently, the risk of triggering hypersensitivity and immunogenic reactions is minimal, providing long-lasting results [5,17]. In addition, ACP Technology ensures the absence of cross-linking agent residues, resulting in a product with an excellent safety profile and minimal risk of immune reactions or adverse events associated with the hydrogel, providing safety and biocompatibility [3,10]. As expected, the reported adverse events included local reactions at the injection site, such as redness, mild swelling, and ecchymosis. Almost all subjects experienced a transient local reaction characterized by redness and mild swelling immediately after the procedure. However, all these reactions resolved spontaneously. This aligns with findings from a pilot study by Alessandrini and Tretyakova, who identified mild bruising and redness as adverse effects of ACP HA and recognized them as an expected consequence of intradermal injection treatments [11]. As described in the literature, erythema and transient edema usually occur immediately after the procedure with all injectable formulations due to local trauma, with erythema lasting several hours and edema lasting several days and, in some cases, up to a week [18]. Ecchymosis, observed in 6 treated sites (5.9%) (Table 4), lasted for a few days and likely resulted from the treatment procedure rather than the product itself. Indeed, as reported by Chiang et al. (2017) such procedures induce microtrauma at the level of the capillaries, which generates microbleeding and the formation of ecchymosis [18].
This study is limited by the small sample size and the lack of a comparison group; however, it provides valuable evidence about the safety and performance of ACP HA. Randomized prospective studies with a larger sample size and a follow-up period of more than 6 months are advisable to confirm these results.
Conclusions
ACP HA is an injectable formulation that has been shown to be safe and well tolerated, providing long-lasting wrinkle reduction and a rejuvenating effect on the skin in the specified areas, including the periocular, perioral and neck regions.
References
- Buck DW, Alam M, Kim JYS (2009) Injectable fillers for facial rejuvenation: a review. J Plast Reconstr Aesthet Surg 62(1): 11-18.
- Guinot C, Malvy DJM, Ambroisine L, Latreille J, Mauger E, et al. Relative Contribution of Intrinsic vs Extrinsic Factors to Skin Aging as Determined by a Validated Skin Age Score. Arch Dermatol 138(11): 1454-1460.
- Wongprasert P, Dreiss CA, Murray G (2022) Evaluating hyaluronic acid dermal fillers: A critique of current characterization methods. Dermatol Ther 35(6): e15453.
- Kim JE, Sykes J (2011) Hyaluronic Acid Fillers: History and Overview. Facial Plast Surg 27(06): 523-528.
- Guarise C, Barbera C, Pavan M, Panfilo S, Beninatto R, et al. (2019) HA-based dermal filler: Downstream process comparison, impurity quantitation by validated HPLC-MS analysis, and in vivo residence time study. J Appl Biomater Funct Mater 17(3): 228080001986707.
- Manuskiatti W, Maibach HI (1996) Hyaluronic Acid and Skin: Wound Healing and Aging. Int J Dermatol. 35(8): 539-544.
- Jones D (2011) Volumizing the Face with Soft Tissue Fillers. Clin Plast Surg 38(3): 379-390.
- Tezel A, Fredrickson GH (2008) The science of hyaluronic acid dermal fillers. J Cosmet Laser Ther 10(1): 35-42.
- Mansouri Y, Goldenberg G (2016) Update on Hyaluronic Acid Fillers for Facial Rejuvenation. Cosmet Dermatol 96(2): 85-88.
- Pluda S, Pavan M, Galesso D, Guarise C (2016) Hyaluronic acid auto-crosslinked polymer (ACP): Reaction monitoring, process investigation and hyaluronidase stability. Carbohydr Res 433: 47-53.
- Alessandrini A, Tretyakova K (2018) The Rejuvenating Effect and Tolerability of an Auto-Cross-Linked Hyaluronic Acid on Décolletage: A Pilot Prospective Study. Aesthetic Plast Surg 42(2): 520-529.
- Fitzpatrick TB (1988) The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol 124(6): 869-871.
- Glogau RG (1996) Aesthetic and anatomic analysis of the aging skin. Semin Cutan Med Surg. 15(3):134-138.
- Day DJ, Littler CM, Swift RW, Gottlieb S (2004) The Wrinkle Severity Rating Scale: A Validation Study. Am J Clin Dermatol 5(1): 49-52.
- Narins RS, Brandt F, Leyden J, Lorenc ZP, Rubin M, et al. (2003) A Randomized, Double-Blind, Multicenter Comparison of the Efficacy and Tolerability of Restylane Versus Zyplast for the Correction of Nasolabial Folds. Dermatol Surg 29(6): 588-595.
- Alessandrini A, Fino P, Giordan N, Amorosi V, Scuderi N (2015) Evaluation of a new hyaluronic acid dermal filler for volume restoration. J Cosmet Laser Ther 17(6): 335-342.
- Krauss MC (1999) Recent advances in soft tissue augmentation. Semin Cutan Med Surg 18(2): 119-128.
- Chiang YZ, Pierone G, Al‐Niaimi F (2017) Dermal fillers: pathophysiology, prevention and treatment of complications. J Eur Acad Dermatol Venereol 31(3): 405-413.






























