Satisfaction of Patients with Androgenetic Alopecia from a Topical Treatment with TH07 - A New Novel Combination of Substances that Safely Encourage Hair Growth
Houfar Sekhavat1, Peter Ford2, Ariana Nateghi3, Sara Bar Yehuda4*
1Triplehair Inc.Canada, Canada
2PharmD, Ford’s Family Pharmacy and Wellness Center, Canada
3Triplehair Inc.Canada, Candidate MD, Sherbrooke, Canada
4Triplehair Inc.Canada, 21 Arbel St. Rishon Le Zion, Israel
Submission: December 18, 2024;Published: January 05, 2024
*Corresponding author: Sara Bar Yehuda, Triplehair Inc.Canada, 21 Arbel st. Rishon Le Zion, Israel, 75474, Email: sara@biomedwrite.co.il
How to cite this article: Houfar S, Peter F, Ariana N, Sara Bar Y. Satisfaction of Patients with Androgenetic Alopecia from a Topical Treatment with TH07 - A New Novel Combination of Substances that Safely Encourage Hair Growth. JOJ Dermatol & Cosmet. 2024; 5(45: 555671. DOI: 10.19080/JOJDC.2024.05.555671
Abstract
Introduction: Hair loss can reduce body image satisfaction, leading to fear of social rejection and a perception of losing social attractiveness. Androgenetic alopecia (AGA) is a hair loss condition that primarily affects the top and front of the scalp. Current treatment options for AGA include the U.S. Food and Drug Administration approved topical Minoxidil and oral Finasteride. In addition, many therapies have been suggested for the treatment of AGA, however the outcomes of those treatments vary widely, with limited or no satisfaction being reported. The Triple Hair Inc. company designed the TH07 formula, which contains 3 drugs that have already demonstrated their ability to induce hair re-growth, while still being safe in animal models and human clinical trials. The goal of the current trial was to evaluate the satisfaction of AGA patients with the TH07 treatment.
Methods: The TH07 product comprises of 5% Minoxidil, 0.1% Finasteride and 0.03% Latanoprost dissolved in a solvent vehicle. The product was sprayed topically once daily on the targeted area. Participants were recruited from privet dermatology clinics in Moncton and the surrounding area, New Brunswick, Canada. The patients were asked to complete a questionnaire in which they had to report about the baldness and appearance of their hair, adverse events, compliance and (4) satisfaction upon the TH07 treatment.
Results: Most of the patients agreed that since they started the treatment their hair loss was controlled and that their baldness and the appearance of their hair had improved, they did not suffer from any adverse event and that the treatment was acceptable and tolerable, that the drug was comfortable and easy to use and that the treatment with the TH07 met their expectations. Interestingly, females were less satisfied with the TH07 treatment than males.
Conclusion: Loss of hair has been found to be associated with impaired body image and self-esteem, thereby accompanied by psychological stress. In the current study AGA patients who were treated with a new topical formula – TH07 were highly satisfied with the efficacy and tolerability of the treatment. Interestingly, women were less satisfied with the treatment than man.
Keywords: Androgenic Alopecia; topical Treatment; Finasteride; Latanoprost; Minoxidil; combination; Satisfaction
Introduction
Hair growth on the head has an important role in heat insulation, cooling and protecting the scalp against ultra-violet radiation [1]. Additionally, hair plays an important role in human social, psychological and sexual communication, reflecting the person’s status, personality, and perception [2]. Androgenetic alopecia (AGA) is characterized by follicular miniaturization in a patterned hair loss occurring due to systemic androgen and genetic factors [3]. The incidence of AGA increases with age and affects up to 50% of all men by age 50 and 80% by age 80 [4]. Hair loss can reduce body image satisfaction, leading to fear of social rejection and a perception of losing social attractiveness [5] Aukerman et al., screened a total of 13 studies (dated from 1992 to 2021) and found that AGA patients express feelings of anxiousness, helplessness, low self-esteem, and fear of increased hair loss. This psychosocial stress leads to a decline in the quality of life [6]. Furthermore, AGA was found to be associated with somatisation, depression, and impaired sexual function [7]. Tas et al., found that among 353 patients with AGA, the risk of psychosexual symptoms correlated with the heightened severity of hair loss [8]. Interestingly, a higher risk of developing psychological problems was exhibited by young unmarried males with AGA.6 Goldberg– Huxley et al., discovered that those seeking specialist consulting regarding their AGA, exhibited a more impaired quality of life compared to those who do not [9]. Actually, the most important factors that patients reported concerning their expectations of the suggested treatment for their hair loss were hair restoration, the onset of results and the cost of treatment. Surprisingly, the potential side effects were less important than promoting hair growth or the progression of alopecia [10].
Current treatment options for AGA include the U.S. Food and Drug Administration approved topical Minoxidil and oral Finasteride. In addition, hormonal therapy, hair transplantation and some new treatment modalities of platelet-rich plasma injections and low-level laser light therapies, as well over-the-counter ointments, soaps, and shampoos have been suggested for the treatment of AGA. Unfortunately, the outcomes of those treatments vary widely, with limited or no satisfaction being reported. Thus, there is still a need for new, more efficacious, convenient to use and safe treatments [11-13].
Finasteride is a DTH suppressing 5-alfa-reductase inhibitor [14]. Latanoprost is a prostaglandin F2α analogues; [15] and Minoxidil, is a pyrimidine derivative [16]. Data from various animal model experiments and clinical studies have demonstrated that topical application of the drugs was able to induce hair growth and was well tolerated [17-19]. Unfortunately, those drugs’ efficacy may be considered insufficient, the beneficial results evident after continuous topical application of at least 4 months and up to 12 months and discontinuation of the treatment results with recurrence of hair loss. Consequently, attempts have been invested to attain improved outcomes using different combinations of the drugs to treat AGA. Such combinations are topical Finasteride + topical Minoxidil20-23 and topical Latanoprost + topical Minoxidil [24]. The data from those studies clearly demonstrated that the combination regimen was superior to either of the monotherapies.
The Triple Hair Inc. company designed the TH07 formula, which contains 0.1% Finasteride, 0.03% Latanoprost and 5% Minoxidil, to be topically applied for the treatment of AGA patients. The efficacy and safety of this new treatment has already been tested in a proof-of-concept study consisting of 34 AGA patients (unpublished data). The TH07 treatment resulted in hair re-growth, while still being safe, compared with each of its components used as a monotherapy. The goal of the current trial was to evaluate the satisfaction of AGA patients with the TH07 treatment.
Methods
The drug
The TH07 product and all the ingredients were developed and manufactured by Triple Hair in a cGMP manufacturing facility. The product comprises 5% Minoxidil, 0.1% Finasteride and 0.03% Latanoprost dissolved in a solvent vehicle comprising absolute alcohol, propylene glycol and diethyl glycol. The concentrations of the 3 drugs were selected according to current usage (either in AGA or in other indications) and are considered to be safe and well tolerated. The product was packaged in 60 mL opaque white bottles filleted with metered pumps. The pumps were calibrated to dispense 100μL per spray. The test product was stored at temperatures between 150C to 300C.
Patients
Participants were recruited from privet dermatology clinics in Moncton and the surrounding area, New Brunswick, Canada. The only pharmacy authorized to dispense the TH07 was the Ford Apothecary (Moncton, New Brunswick, Canada). The drug was applied once a day to the targeted area. Patients were called by the Ford pharmacy staff. They were asked to go online to fill out the questionnaire and digitally sign it. They were informed that these results could be used for the approval of the drug by the Canadian health authorities).
Questionnaire
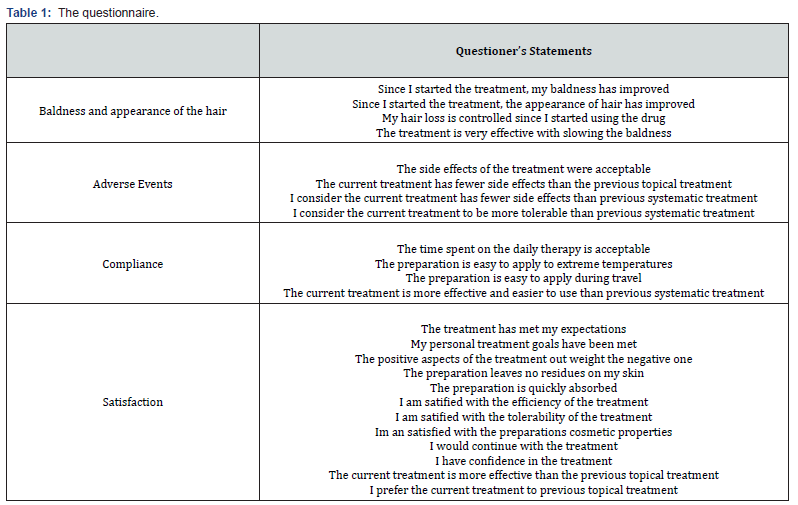
The questionnaire was divided into 4 sections describing: (1) the baldness and appearance of the hair, (2) adverse events, (3) compliance and (4) satisfaction (Table 1). The various questions were selected based on the “Treatment Satisfaction Questionnaire for Medication”.25 Participants had to complete in the questionnaire by ticking one of the following statements for each question: “Does not apply to me” scored as 0, “Strongly disagree” – scored as -1, “Disagree” - scored as 2, “Agree”- scored as 3 and “Strongly agree” - scored as 4. In addition, the sex and starting date of treatment commencement were collected.
Statistical Analysis
The patient’s answers to the questionnaire were analyzed separately and in sections. Comparing means of percent’s scores between different groups were performed using 2 tailed t-test for independent samples. Chi-Square Goodness of Fit Test was calculated to compare gender distributions of answers. Pearson correlation coefficient was performed to assess the linear correlation between the time between treatment initiation and the completion of the questioner to the mean scores of the questionnaire. Differences considered as statistically significant at Pv of < 0.05.
Results
Out of the 160 TH07-treated patients,35 competed the questioner, most of them were males (27/35, 77.1%). The mean time between treatment initiation and the completion of the questioner was 20.7 months (ranging between 0.6-96, median 14.3).
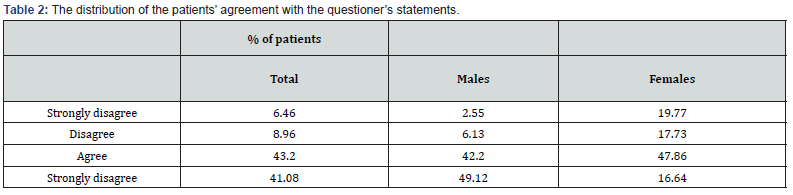
Most patients agreed or strongly agreed with the questionnaire’s statements (43 % and 41% (vs. disagree or strongly disagree (9 % and 6.5%, respectively, P<0.001). Interestingly, more males agreed or strongly agreed with the questioner’s statements compared to females (91.32% vs. 62.5%, respectively), while fewer males strongly disagreed or disagreed with the questionnaire’s statements compared to females (8.68% vs. 37.5%, respectively) (Table 2).
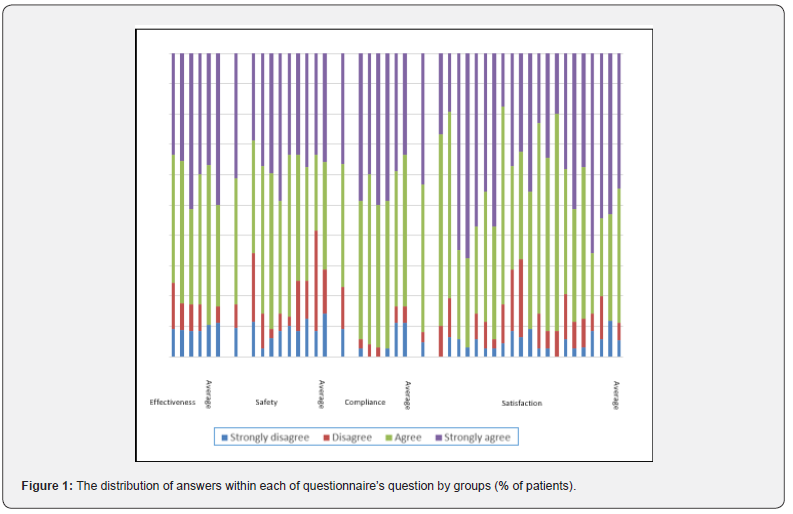
The same trend was observed when examining the distribution of the answers according to the different sections of the questionnaire (Figure 1). Most of the patients agreed or strongly agreed (37% and 31%, respectively) that since they started the treatment their hair loss was controlled and that their baldness and the appearance of their hair had improved. Forty-three percent of the patients agreed vs 14% who disagreed that they did not suffer from any adverse event and that the treatment was acceptable and tolerable. Half of the patients agreed that the drug was comfortable and easy to use, while only 5% disagreed. Most patients agreed (43%) or strongly agreed (33%) that the treatment with the TH07 met their expectations; they were satisfied with the efficacy and tolerability of the treatment, had confidence in this treatment and would like to continue with it (Table 3).
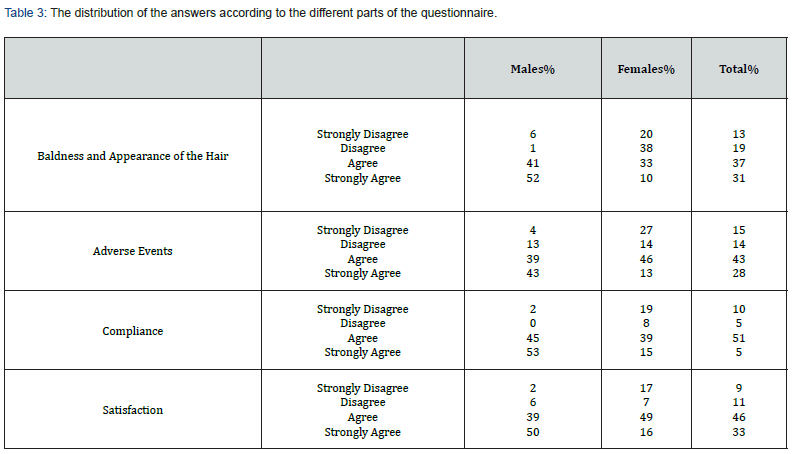
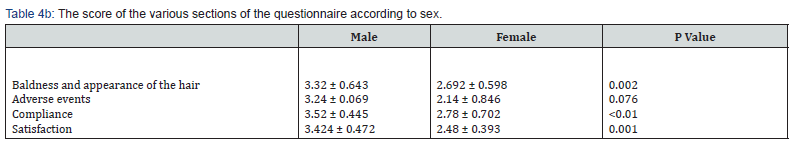
Interestingly, while 41% and 52% of the males agreed or strongly agreed that there was an improvement in their baldness and in the hair appearance, only 33% agreed and 10% of the females strongly agreed. Most males (43%) vs 17% of females strongly agreed that the treatment was acceptable and more tolerable than any previous treatments. While 53% of the men strongly agreed that TH07 was easy to apply only 15% of the females did so. Also, 50% of the males were satisfied with the TH07 treatment (strongly agreed), whereas only 16% of the females strongly agreed that they were satisfied (Table 3). Chi square goodness of fit P<0.001 for distribution differences between gender in all 4 question’s groups.
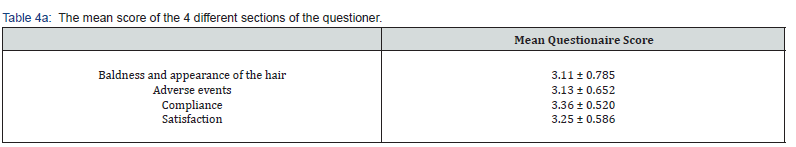
The mean of the questionnaire scoring was 3.22 ± 0.58. The mean of the questionnaire scoring by males was significantly higher than that of females (3.38 ± 0.48 vs. 2.69 ± 0.6 respectively, P=0.002). The score of the 4 different sections of the questionnaire also ranked high (ranging from 3.11–3.36) There was a significant difference between the mean score of the baldness and the hair’s appearance of vs. the compliance score (p=0.049) and between the adverse events score vs. the compliance score (p=0.007) (Table 4a). The mean score of baldness and appearance of the hair, compliance and satisfaction was significantly higher among males compared to women (Table 4b).
The Pearson correlation coefficient test revealed that there was no linear correlation between the time between treatment initiation and the completion of the questioner to the mean scores of the questionnaire was not significant (P=0.519, r=-0.113).
Discussion
The attitude of the AGA patients toward their hair loss could harm their quality of life due to an impaired self-image and low self-esteem. Treatment options are currently limited, and a significant length of time is required to reach satisfactory outcomes. TH07, developed by Triple Hair Inc. company, is a combination of three drugs (Finasteride, Latanoprost and Minoxidil) which have already demonstrated their efficacy and safety as a treatment for AGA in numerous animal models as well as in clinical studies. It is suggested that this combination affects various mechanisms of action (such as anti-androgenic effects, increase the levels of growth factors and cell cycle regulators and increase oxygen and blood supply to the dermal papilla cells) which are involved in the process of hair growth [14,26-28]. Activation of all those mechanisms of action simultaneously may result in an improved outcome. The satisfaction of AGA patients upon treatment with topical administration of Minoxidil was monitored in some studies. Topical foam of 5% Minoxidil was applied to males with AGA for 16 weeks, and 12 continued up to 24 weeks. At weeks 16 and 24, 50.2% and 75.0% of the participants were very satisfied, respectively, with the efficacy of the treatment. The patients were also very satisfied with the drug’s convenience of use [29]. Another study revealed that patients 33.3% and 25% of the patients treated with 5% or 10% of Minoxidil for 6 months, respectively, were moderately satisfied, 55.6% and 62.5% (respectively) well satisfied [30].
Topical 5% Minoxidil+oral 1 mg Finasteride or topical 5% Minoxidil+topical 0.1 Finasteride were applied to 50 patients with AGA for 12 months. Although, the clinical outcome was slightly higher, but significant, in the 2 topical treatment group compared to the group treated with the oral Finasteride, the improvement in the quality of life was significantly higher among those who were treated with topical Minoxidil and Oral Finasteride. Nevertheless, it is important to mention that 39 side effects were reported by the patients in the topical Minoxidil and Oral Finasteride, while only 12 side effects were reported by the group who had only topical treatment. The side effects of the oral Finasteride group included beside dermatological local symptoms, erectile and ejaculatory dysfunction, anxiety/depression, and mood changes, while the topical treatment group exhibited only dermatological local symptoms side effects [31]. In another study patients were randomly assigned to receive either topical 5% Minoxidil+1 mg Finasteride tablets or topical 5% Minoxidil 5%+topical 0.1% Finasteride combination with placebo tablets, for 6 months. The combination of topical Minoxidil+Finasteride was safe and equally efficacious as the topical Minoxidil+oral Finasteride treatment, both in hair growth and patients’ satisfaction [32]. Thus, topical administration of drugs combinations could be an alternative treatment to the topical Minoxidil plus oral Finasteride which may result in adverse events associated with administration of oral Finasteride.
In the current study the level of satisfaction from the TH07 treatment with was explored among AGA patients. Most patients were highly satisfied with the efficacy and tolerability of the treatment. They were confident in the treatment and expressed interest in continuing with it. They found the current treatment was more effective than the previous topical treatments and preferred it. They reported that the drug was easy to handle and use and therefore exhibited a high rate of compliance with the treatment. However, patients stated that these side effects were fewer than the topical or the systemic previous treatments they had for the AGA.
It is well established that the psychological stress upon hair loss is greater among women compared with men. Women believe that their hair reflects their beauty, femininity, and attractiveness. Hunt et al., showed that about 40% of women with alopecia were reported to have marital problems and 63% claimed to have career-related issues.33 Zac et al., found that of e 31 women with AGA, 54.8% declared that their hair loss influenced their social life and in 87.1% it dictated their choice of hair cut/hairstyl.34 Another study demonstrated that women were significantly more willing to pay for a hypothetical cure of their disease compared men [35]. This difference could be attributed to the fact that women are more conscious about their cosmetic and physical appearance than men. Zhuang et al., treated 31 females with a topical 2% Minoxidil solution for 12 months. A significant improvement in the dermatological quality of life questionnaire score upon the Minoxidil treatment compared to the score before treatment initiation was noted [36] Zac et al., conducted a study aimed to evaluate satisfaction and quality of life among women with AGA using 5% topical Minoxidil for at least six months. Satisfaction with treatment and comfort was reported by 83.9% of the participants [34].
The data from our study revealed that females were less satisfied with the TH07 treatment than males. Less of the females agreed or strongly agreed that there was an improvement in their baldness and hair appearance, that the treatment was tolerable and easy to use and that they were satisfied with the treatment. Also, the mean score of the questioner, as well as the mean score of each of the individual sections was lower among the females compared to the score of the males This could be attributed to the fact that psychological stress upon hair loss is higher in women compared with men. In addition, hair growth of a few millimeters can be sufficient for men, but it is not enough for women who are eager for longer hair.
Conclusion
Loss of hair has been found to be associated with impaired body image and self-esteem, thereby accompanied by psychological stress. In the current study AGA patients who were treated with a new topical formula -TH07 were highly satisfied with the efficacy and tolerability of the treatment. Interestingly, women were less satisfied with the treatment than man.
Limitations
One limitation of our study in the small sample size. In addition, although significant differences in the satisfaction with the treatment was observed between the males and the females, the number of women was much lower than the men, thereby, could not produce a satisfied power for the analysis.
References
- Jablonski NG, Chaplin (2010) Colloquium paper: human skin pigmentation as an adaptation to UV radiation. Proc Natl Acad Sci U S A 107 Suppl(Suppl 2): 8962-8968.
- Wang X, Xiong C, Zhang L, Yang B, Rongfang Wei, et al. (2018) Psychological assessment in 355 Chinese college students with androgenetic alopecia. Medicine (Baltimore) 97(31): e11315.
- Sinclair R, Torkamani N, Jones L (2015) Androgenetic alopecia: new insights into the pathogenesis and mechanism of hair loss. F1000Research 4(F1000 Faculty Rev): 585.
- Hamilton jb (1951) Patterned loss of hair in man; types and incidence. Ann n y acad sci 53(3):708-728.
- Williamson D, Gonzalez M, Finlay AY (2001) The effect of hair loss on quality of life. J Eur Acad Dermatol Venereol. 2001;15(2):137-139.
- Aukerman EL, Jafferany M (2023) The psychological consequences of androgenetic alopecia: A systematic review. J Cosmet Dermatol. 22(1): 89-95.
- Sinikumpu SP, Jokelainen J, Auvinen J, Timonen M HL (2021) Association between psychosocial distress, sexual disorders, self-esteem and quality of life with male androgenetic alopecia: a population-based study with men at age 46. BMJ Open 11: e049855.
- Tas B, Kulacaoglu F, Belli H, Altuntas M (2018) The tendency towards the development of psychosexual disorders in androgenetic alopecia according to the different stages of hair loss: a cross-sectional study. An Bras Dermatol. 2018;93(2): 185-190.
- Gupta AK, Mays RR, Versteeg SG, Shear NH, Piguet V, et al. (2019) Efficacy of Off-Label Topical Treatments for the Management of Androgenetic Alopecia: A Review. Clin Drug Investig 39(3): 233-239.
- Lulic Z, Inui S, Sim WY, Kang H, Seong Choi G, et al. (2017) Understanding patient and physician perceptions of male androgenetic alopecia treatments in Asia-Pacific and Latin America. J Dermatol 44(8): 892-902.
- Pachar S, Chouhan C, Rao P, Kachhawa D, Singh H, et al. (2022) A Comparative Study of Efficacy of 5% Minoxidil and 5% Minoxidil Plus Platelet-Rich Plasma in Same Patient for Treatment of Androgenetic Alopecia. J Cutan Aesthet Surg 15(1): 71-76.
- Olsen EA, Dunlap FE, Funicella T, Koperski JA, Swinehart JM, et al. (2022) A randomized clinical trial of 5% topical minoxidil versus 2% topical minoxidil and placebo in the treatment of androgenetic alopecia in men. J Am Acad Dermatol 47(3): 377-385.
- Rattanachitthawat N, Pinkhien T, Opanasopit P, Ngawhirunpat T, Chanvorachote P (2019) Finasteride Enhances Stem Cell Signals of Human Dermal Papilla Cells. In Vivo 33(4): 1209-1220.
- Mella JM, Perret MC, Manzotti M, Catalano HN, Guyatt G (2010) Efficacy and safety of finasteride therapy for androgenetic alopecia: a systematic review. Arch Dermatol 146(10):1141-1150.
- Johnstone MA (1997) Hypertrichosis and increased pigmentation of eyelashes and adjacent hair in the region of the ipsilateral eyelids of patients treated with unilateral topical latanoprost. Am J Ophthalmol 124(4): 544-547.
- Messenger AG, Rundegren J (2004) Minoxidil: mechanisms of action on hair growth. Br J Dermatol 150(2): 186-194.
- Goren A, Naccarato T (2018) Minoxidil in the treatment of androgenetic alopecia. Dermatol Ther 31(5): e12686.
- Suchonwanit P, Iamsumang W, Leerunyakul K (2022) Topical finasteride for the treatment of male androgenetic alopecia and female pattern hair loss: a review of the current literature. J Dermatolog Treat 33(2): 643-648.
- Blume-Peytavi U, Lönnfors S, Hillmann K, Garcia Bartels N (2012) A randomized double-blind placebo-controlled pilot study to assess the efficacy of a 24-week topical treatment by latanoprost 0.1% on hair growth and pigmentation in healthy volunteers with androgenetic alopecia. J Am Acad Dermatol 66(5): 794-800.
- Tanglertsampan C (2012) Efficacy and safety of 3% minoxidil versus combined 3% minoxidil / 0.1% finasteride in male pattern hair loss: a randomized, double-blind, comparative study. J Med Assoc Thai 95(10): 1312-1316.
- Sheikh S, Ahmad A, Ali SM, Ahmad MU, Paithankar M, et al. (2015) A New Topical Formulation of Minoxidil and Finasteride Improves Hair Growth in Men with Androgenetic Alopecia. J Clin Exp Dermatol Res. 6(1): 2-6.
- Suchonwanit P, Srisuwanwattana P, Chalermroj N KS (2018) A randomized, double-blind controlled study of the efficacy and safety of topical solution of 0.25% finasteride admixed with 3% minoxidil vs. 3% minoxidil solution in the treatment of male androgenetic alopecia. J Eur Acad Dermatol Venereol 32: 2257-2263.
- Marotta JC, Patel G, Carvalho M, Blakeney S (2020) Clinical Efficacy of a Topical Compounded Formulation in Male Androgenetic Alopecia: Minoxidil 10%, Finasteride 0.1%, Biotin 0.2%, and Caffeine Citrate 0.05% Hydroalcoholic Solution. Int J Pharm Compd 24(1): 69-76.
- Bloch LD, Escudeiro CC, Sarruf FD, Valente NYS (2018) Latanoprost and minoxidil: Comparative double-blind, placebo-controlled study for the treatment of hair loss. Surg Cosmet Dermatology 10(1): 39-43.
- Atkinson MJ, Kumar R, Cappelleri JC, Hass SL. (2005) Hierarchical construct validity of the treatment satisfaction questionnaire for medication (TSQM version II) among outpatient pharmacy consumers. Value Heal J Int Soc Pharmacoeconomics Outcomes Res 8(Suppl 1): S9-S24.
- Hsu C-L, Liu J-S, Lin A-C, Yang C-H, Chung W-H, et al. (2014) Minoxidil may suppress androgen receptor-related functions. Oncotarget 5(8): 2187-2197.
- Lachgar S, Charveron M, Gall Y, Bonafe JL (1998) Minoxidil upregulates the expression of vascular endothelial growth factor in human hair dermal papilla cells. Br J Dermatol 138(3): 407-411.
- Husain S, Jafri F, Crosson CE (2005) Acute effects of PGF2alpha on MMP-2 secretion from human ciliary muscle cells: a PKC- and ERK-dependent process. Invest Ophthalmol Vis Sci 46(5): 1706-1713.
- Hasanzadeh H, Nasrollahi SA, Halavati N, Saberi M, Firooz A (2016) Efficacy and safety of 5% minoxidil topical foam in male pattern hair loss treatment and patient satisfaction. Acta dermatovenerologica Alpina, Pannonica, Adriat 25(3): 41-44.
- Ghonemy S, Alarawi A, Bessar H (2021) Efficacy and safety of a new 10% topical minoxidil versus 5% topical minoxidil and placebo in the treatment of male androgenetic alopecia: a trichoscopic evaluation. J Dermatolog Treat 32(2): 236-241.
- Rai PB, Khushwaha P, Jain N GS (2018) Comparing the therapeutic efficacy of topical minoxidil and finasteride with topical minoxidil and oral finasteride in androgenetic alopecia: a randomized trial. Int J Res Dermatol 4(3): 386-390.
- Datta D, Saha A, Gharami RC, Bandyopadhyay D DN (2021)Effectiveness of Topical Minoxidil (5%) Plus Topical Finasteride (0.1%) Fixed-Dose Combination Versus Topical Minoxidil (5%) Plus Oral Finasteride (1 Mg/Day) in Grade II-IV Androgenetic Alopecia: A Randomized, Double Blind Clinical Trial. Asian J Res Dermatological Sci 4(1): 6-18.
- Dhami L (2021) Psychology of Hair Loss Patients and Importance of Counseling. Indian J Plast Surg Off Publ Assoc Plast Surg India 54(4): 411-415.
- Zac RI, da Costa A (2021) Patient Satisfaction and Quality of Life Among Adult Women with Androgenetic Alopecia Using 5% Topical Minoxidil. J Clin Aesthet Dermatol 14(5): 26-30.
- Mubki T, Dayel S, AlHargan A, AlGhamdi K AA (2019) Quality of life and willingness‑to‑pay in patients with androgenetic alopecia. Egypt J Dermatol Venerol 39(1): 31–36.
- Zhuang X S, Zheng YY, Xu JJ, Fan WX (2013) Quality of life in women with female pattern hair loss and the impact of topical minoxidil treatment on quality of life in these patients. Exp Ther Med 6(2): 542-546.






























