- Research article
- Abstract
- Background and Introduction
- Method
- Review
- Type IV hypersensitivity
- Urticarial cADRs
- Serum Sickness-Like Reaction
- Bullous Eruptions
- Drug-Induced Pemphigus (DIP)
- Drug Induced Linear IgA Bullous Dermatosis (LABD)
- Stevens-Johnson Syndrome (SJS) and Toxic Epidermal Necrolysis (TEN)
- Pustular cADRs
- Acute Generalized Exanthematous Pustulosis (AGEP)
- Acute Localized Exanthematous Pustulosis (ALEP)
- Drug Induced Sub Corneal Pustular Dermatosis (SPD)
- Miscellaneous
- Drug Induced Alopecia
- Drug-Induced Photosensitivity
- Drug-Induced Vasculitis
- Severe Cutaneous Adverse Drug Reactions (ScADRs)
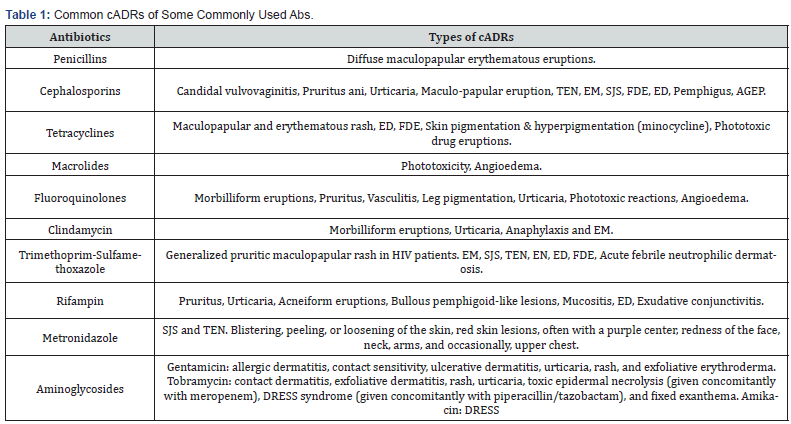
- Cutaneous Adverse Drug Reactions to Some Common Antibiotics
- Discussion
- Conclusion
- References
Cutaneous Adverse Drug Reactions to Antibiotics: How Much is the Extent of the Problem?
Badar Uddin Umar1* and Zeenat Un Nisa2
1Coordinator, Research Integrations, Center for Biomedical Research & Training (CBRT), Bangladesh
2MD, International Medical Graduate (IMG)
Submission: July 07, 2023;Published: August 25, 2023
*Corresponding author: Badar U Umar, Coordinator, Research Integration, Center for Biomedical Research & Training (CBRT), Bangladesh, Email: badarumar@gmail.com
How to cite this article: Badar Uddin U, Zeenat Un N. Cutaneous Adverse Drug Reactions to Antibiotics: How Much is the Extent of the Problem?. JOJ Dermatol & Cosmet. 2023; 5(4): 555666. DOI: 10.19080/JOJDC.2023.05.555666
- Research article
- Abstract
- Background and Introduction
- Method
- Review
- Type IV hypersensitivity
- Urticarial cADRs
- Serum Sickness-Like Reaction
- Bullous Eruptions
- Drug-Induced Pemphigus (DIP)
- Drug Induced Linear IgA Bullous Dermatosis (LABD)
- Stevens-Johnson Syndrome (SJS) and Toxic Epidermal Necrolysis (TEN)
- Pustular cADRs
- Acute Generalized Exanthematous Pustulosis (AGEP)
- Acute Localized Exanthematous Pustulosis (ALEP)
- Drug Induced Sub Corneal Pustular Dermatosis (SPD)
- Miscellaneous
- Drug Induced Alopecia
- Drug-Induced Photosensitivity
- Drug-Induced Vasculitis
- Severe Cutaneous Adverse Drug Reactions (ScADRs)
- Cutaneous Adverse Drug Reactions to Some Common Antibiotics
- Discussion
- Conclusion
- References
Abstract
Medication-related harm or adverse drug reaction is a great global health concern today. They are frequent and, most often, devastating. With the rising incidences of drug-related adverse outcomes, it is imperative that clinicians have the knowledge and understanding of these occurrences to prevent, diagnose, and manage them properly. Skin is a very commonly affected organ system, and incidences of cutaneous adverse drug reactions are between 1-5% depending on the geographic location. Up to 60% or more of all adverse drug reactions may represent cutaneous reactions. These may fall into either of the dose-dependent or dose-independent categories. Being the most prescribed and utilized drug group, antibiotics have been implicated as one of the major drug groups causing cutaneous drug reactions. There is ample evidence that antibiotics cause severe and non-severe cutaneous adverse drug reactions. A review of the present literature was done using MEDLINE/PubMed, Google Scholar, and other search engines to gather evidence to find how extensive the problem is and how much the burden is. Which will enlighten clinicians from all backgrounds and medical students and help them deal with cutaneous adverse drug reactions more prudently.
Keywords: Adverse drug reactions; Cutaneous drug reactions; Antibiotics; Antimicrobials; Skin eruptions; Hypersensitivity reactions
- Research article
- Abstract
- Background and Introduction
- Method
- Review
- Type IV hypersensitivity
- Urticarial cADRs
- Serum Sickness-Like Reaction
- Bullous Eruptions
- Drug-Induced Pemphigus (DIP)
- Drug Induced Linear IgA Bullous Dermatosis (LABD)
- Stevens-Johnson Syndrome (SJS) and Toxic Epidermal Necrolysis (TEN)
- Pustular cADRs
- Acute Generalized Exanthematous Pustulosis (AGEP)
- Acute Localized Exanthematous Pustulosis (ALEP)
- Drug Induced Sub Corneal Pustular Dermatosis (SPD)
- Miscellaneous
- Drug Induced Alopecia
- Drug-Induced Photosensitivity
- Drug-Induced Vasculitis
- Severe Cutaneous Adverse Drug Reactions (ScADRs)
- Cutaneous Adverse Drug Reactions to Some Common Antibiotics
- Discussion
- Conclusion
- References
Background and Introduction
Using medications in patient care is a cornerstone of modern therapies, which brings with it the risk of unwanted medication-related harm or adverse effects (AEs) to drugs [1,2], and not having a causal relationship with the treatment [3]. Up to one in ten patients experience these negative outcomes of medications. Annual cost related to medication-related herm was estimated to be about $42 billion USD. WHO has launched programs targeting reduction of medication related harms [4]. A noxious and unintended response to medicinal products occurring at therapeutic doses is called adverse drug reaction (ADR) [5-7]. ADRs are common in healthcare settings and now pose a global public health threat [5]. They cause morbidity and mortality beside incurring additional cost [8]. Most ADRs are preventable [6,9]. Antibiotics were found to be a main culprit causing cADRs [10,11]. Many of the fatal ADRs are caused by antibiotics (ABs) [5].
Among all types of ADRs, the cutaneous adverse drug reactions (cADRs) are the commonest and may represent up to 30% to 60% of all ADRs [12,3]. Sokolewicz et al., (2021) reported even more higher rates (60-70%) [14]. Incidences of cADRs vary globally, being 1-3% in the developed countries and 2-5% in developing countries [11, 15, 16]. Many cases of cADRs go unreported or remain underreported. Any undesirable structural and/or functional change in the skin or its appendages or mucous membranes are considered as cADR, and this may include drug eruption during drug therapy [13]. Clinical manifestations of the skin, mucous-membranes, and adnexa due to drug or its metabolites are considered as cADR. Majority of these are mild-moderate and are self limiting, but 2-6.7% could become potentially life-threatening like- erythroderma (ED), drug reaction with eosinophilia and systemic symptoms (DRESS), and Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) [17]. cADRs occur most commonly due to antibiotics (ABs) [12, 18-19]. In a systematic review antimicrobial were leading cause of cADRs (45.46%) [20]. A nationally representative public health surveillance done in the US found that over 46% of emergency department (ED) visits were due to adverse drug events related to anticoagulants, antibiotics, and anti-diabetes agents. Antibiotics were responsible for moderate to severe allergic reactions. AB-associated ADRs are common in children too. More than 56% of children <5 years of age experienced ADRs due to ABs and almost 32% of children aged between 6-19 years experienced ADRs due to ABs. Overall, 18.2% patients on antibiotics had moderate to severe allergic reactions, and another 63.3% had the mild allergy [21]. Noel, Sushma, and Guido (2004) [22] reported that, in a prospective hospital-based study, 32% of the cADRs were due to chemotherapeutic agents like ABs [22]. ADRs involving skin is also common in developing countries like- Bangladesh, India [11, 20, 23-26]. A study conducted in the outpatient department of Dhaka Medical College Hospital, Bangladesh found that about 43% of ADRs were caused by ABs, and Skin and Appendages was the second most affected system (32%) next to the GIT (56%) [23]. cADRs like Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) can be fatal and are mostly caused by idiosyncratic drug reactions. A 10-year population-based prospective cohort study done between April 2002 and March 2011 in Ontario, Canada, found that, among 708 hospitalized patients, the incidence rate of SJS was 80% (n=567) and TEN 20% (n=141). About 26% of those patients were admitted to critical care, and 127 (17.9%) died in the hospital [27].
There is a suboptimal understanding of the classification of ADRs among healthcare workers. Differentiating between the classes of ADRs is essential for proper identification and management of them [28]. A review of the existing literature was done to find the extent of AB-associated cADRs. A search of the relevant works of literature was made using MEDLINE/PubMed, Google, Google Scholar, and grey literature, using the keywords such as: "cutaneous adverse drug reaction”, “antibiotics associated ADRs,” “skin and ADRs.” Only articles published in English were included. Also, original research, systematic reviews, meta-analysis, and review articles were considered.
- Research article
- Abstract
- Background and Introduction
- Method
- Review
- Type IV hypersensitivity
- Urticarial cADRs
- Serum Sickness-Like Reaction
- Bullous Eruptions
- Drug-Induced Pemphigus (DIP)
- Drug Induced Linear IgA Bullous Dermatosis (LABD)
- Stevens-Johnson Syndrome (SJS) and Toxic Epidermal Necrolysis (TEN)
- Pustular cADRs
- Acute Generalized Exanthematous Pustulosis (AGEP)
- Acute Localized Exanthematous Pustulosis (ALEP)
- Drug Induced Sub Corneal Pustular Dermatosis (SPD)
- Miscellaneous
- Drug Induced Alopecia
- Drug-Induced Photosensitivity
- Drug-Induced Vasculitis
- Severe Cutaneous Adverse Drug Reactions (ScADRs)
- Cutaneous Adverse Drug Reactions to Some Common Antibiotics
- Discussion
- Conclusion
- References
Method
We searched MEDLINE/PubMed, Google Scholar, Google, and grey literature from 2010 to 2022 but did not exclude commonly cited important articles published before 2010. The search was done using the keywords such as: "cutaneous adverse drug reaction,” “antibiotics associated ADRs”, “skin and ADRs”, and “antimicrobials”. Only articles published in English were included. Also, original research, systematic reviews, meta-analysis, and review articles were considered. We attempted to define various types of cADRs as well as calculate the average and standard deviations for various types of cADRs, both severe and nonservice, besides calculating the frequency of antibiotics or antimicrobials causing cADRs.
- Research article
- Abstract
- Background and Introduction
- Method
- Review
- Type IV hypersensitivity
- Urticarial cADRs
- Serum Sickness-Like Reaction
- Bullous Eruptions
- Drug-Induced Pemphigus (DIP)
- Drug Induced Linear IgA Bullous Dermatosis (LABD)
- Stevens-Johnson Syndrome (SJS) and Toxic Epidermal Necrolysis (TEN)
- Pustular cADRs
- Acute Generalized Exanthematous Pustulosis (AGEP)
- Acute Localized Exanthematous Pustulosis (ALEP)
- Drug Induced Sub Corneal Pustular Dermatosis (SPD)
- Miscellaneous
- Drug Induced Alopecia
- Drug-Induced Photosensitivity
- Drug-Induced Vasculitis
- Severe Cutaneous Adverse Drug Reactions (ScADRs)
- Cutaneous Adverse Drug Reactions to Some Common Antibiotics
- Discussion
- Conclusion
- References
Review
Cutaneous Adverse Drug Reaction and Types
Antibiotics are one of the most prescribed and widely used drug groups [29-30] especially in hospitalized patients [31-32]. Their discovery and availability have revolutionized the treatment of infections since the days of Fleming [33]. Positive impact of AB use is evidenced by the declining rates of global infant and child mortality [34]. Classifying cADRs is difficult and confusing. Depending upon pathophysiology, morphology, time of occurrence and severity they can be classified in many ways. One simple way is clinically dividing them into two broad categories, Type-A (augmented) and Type-B (bizarre) by Rawlins and Thomson (1977) [17, 35, 37]. Drug reaction to ABs may be of either immediate or delayed hypersensitivity type also [38].
Type A reactions
Type-A or augmented ADRs are predictable, dose-dependent reactions, sometimes called side effects or drug toxicities. They are extensions of normal pharmacological actions, mostly due to overdosing, like bradycardia with beta-blockers, and hypoglycemia with insulin. Drug-induced generalized rashes, mucositis, alopecia, nail changes are some examples of Type-A cADRs. These ADRs are mostly transient and are reversible with dose cessation [17,35,37].
Type-B reactions
Also known as bizarre type ADRs and are unpredictable and less common. They are not related to the known pharmacology of the drugs. Often dose-independent, abnormal responses to drugs and commonly known as drug hypersensitivity reactions. Depending upon the mechanism they are subcategorized as non-immunologically mediated and immunologically mediated reactions [17,35,37].
ABs can cause non-immunological and immunological ADRs [18]. Among the two major types of ADRs, dose-dependent, non-immunological ADRs are the commonest (up to 80%) [13] and are usually predictable., e.g., superinfection with broad-spectrum ABs like tetracyclines, ototoxicity with aminoglycosides etc. Others can be unpredictable and idiosyncratic (20%-25%) [13]. Immunologic ADRs comprised of only 5%-10% of all cADRs [12,18] and can be subcategorized into four main types (Type I-IV) (Figure 1) [12, 18, 39] as following:
Type I hypersensitivity reaction
Also known as immediate hypersensitivity reactions as they occur within minutes to hours of drug exposure. Preformed drug (antigen) specific immunoglobulin (IgE) antibodies (Ab) cause mast-cell and/or basophil degranulation releasing inflammatory mediators like histamine leading to rapid vasodilation and increased vascular permeability. The result is drug-induced angioedema, urticaria and anaphylaxis. Previous exposure and sensitization with the drug are required [12,17] (Figure 1).
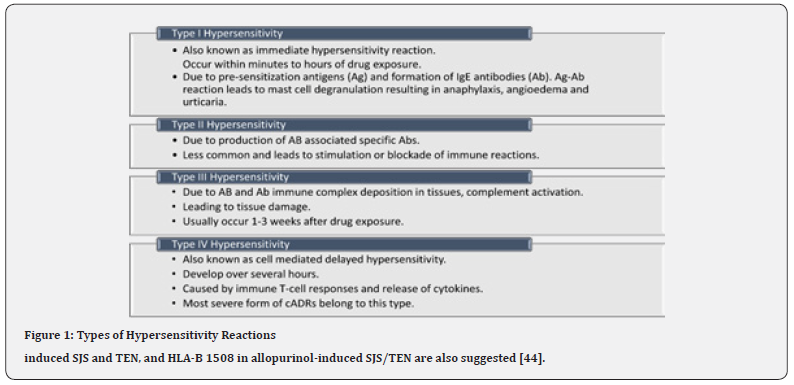
Type II hypersensitivity reaction
Delayed in onset and is less common. Abnormal binding of IgG or IgM Ab to normal tissues leads to complement activation leading to inflammation and tissue damage. Drug-induced bullous pemphigoid is an example [12,17] (Figure 1).
Type III hypersensitivity
Occur 1-3 weeks after drug exposure. There is drug and Ab immune complexes deposition in various tissues like blood vessels, skin, kidneys, joints leading to local tissue damage by activating complement cascades. There may be palpable purpura in the skin [12,17] (Figure 1).
- Research article
- Abstract
- Background and Introduction
- Method
- Review
- Type IV hypersensitivity
- Urticarial cADRs
- Serum Sickness-Like Reaction
- Bullous Eruptions
- Drug-Induced Pemphigus (DIP)
- Drug Induced Linear IgA Bullous Dermatosis (LABD)
- Stevens-Johnson Syndrome (SJS) and Toxic Epidermal Necrolysis (TEN)
- Pustular cADRs
- Acute Generalized Exanthematous Pustulosis (AGEP)
- Acute Localized Exanthematous Pustulosis (ALEP)
- Drug Induced Sub Corneal Pustular Dermatosis (SPD)
- Miscellaneous
- Drug Induced Alopecia
- Drug-Induced Photosensitivity
- Drug-Induced Vasculitis
- Severe Cutaneous Adverse Drug Reactions (ScADRs)
- Cutaneous Adverse Drug Reactions to Some Common Antibiotics
- Discussion
- Conclusion
- References
Type IV hypersensitivity
Type IV hypersensitivity
Also known as cell-mediated delayed hypersensitivity. Mediated by T-cell response, triggered by an inflammatory response to endogenous or exogenous antigen (Ag). Other white blood cells like- monocytes, eosinophils and neutrophils may also be taking part. e.g., contact dermatitis, maculopapular drug reactions, SJS/TEN, acute generalized exanthematous pustulosis AGEP [12,17] (Figure 1).
Morphological Classification of cADRs
Based on clinic-morphological appearance cADRs can be classified into 5 categories: (i) exanthematous, (ii) urticarial, (iii) bullous, (iv) pustular and (v) miscellaneous. As well, some authors have categorized them into simple and complex categories. Any cADR that is mostly limited to the skin and/or mucosa are simple type. They hardly involve systemic tissues. When any cADR involves other systems of the body besides skin, they are called complex. They are characterized by fever, malaise, lymphadenopathy, tachycardia, hypotension, joint-pain, respiratory difficulty, kidney, and liver effects [17].
Exanthematous cADRs
The most common type of drug eruptions (>90%). A heterogeneous type including- (a) morbilliform eruptions (ME), (b) symmetric drug-related intertriginous and flexural erythema/exanthem (SDRIFE), (c) drug-induced hypersensitivity syndrome (DHS) or drug reaction with eosinophilia and systemic symptoms (DRESS), (d) drug-induced erythroderma or exfoliative dermatitis (ED), (e) contact dermatitis, (f) leukocytoclastic vasculitis [12, 17, 40-41].
Morbilliform Eruptions
This is the most common type of AB-induced cADR. Another name is exanthem [41]. Characterized by small, diffuse, fine pink to red macules and papules, hence called maculopapular eruption. It may coalesce into patches and plaques. Rarely vesicles and pustules [40] may appear. Usually, appear within 7-14 days of drug exposure and stay for 5-10 days and appear more early on re-exposure. They are pruritic, start as symmetrical involvement on the trunk and then may spread to the proximal extremities as well distal parts. It can be widespread but does not involve mucosa normally. Some systemic features like fever, itchiness, and leukocytosis or lymphopenia may be present in some cases. The exact pathology is not known. Overexpression of certain cytokines like interleukin (IL-5 and IL-13), and eosinophilia is evident in affected skin. As well, perforin and granzyme B-like molecules may be found. The commonest causative agents are antibiotics, then antiepileptics and NSAIDs [12,17,40].
Symmetric Drug-Related Intertriginous and Flexural Erythema/Exanthem (SDRIFE):
This reaction is also recognized as baboon syndrome. It is a delayed hypersensitivity reaction. There are pruritic, erythematous skin eruptions, especially on the flexural intertriginous areas [42] like inguinal and/or gluteal regions. Well-demarcated lesions with eczema, scaling or desquamation of affected skin are common features. Penicillin and cephalosporins are the causative agents in about 50% of the cases. Clindamycin, erythromycin, nystatin, fluconazole, and metronidazole may also cause SDRIFE. The precise mechanism is unclear, but it’s believed that there is a type-IV hypersensitivity reaction involved, which is evidenced by CD4+ T cell infiltration. It is a self-limiting condition and there is no systemic involvement [12, 17].
Drug-Induced Hypersensitivity Syndrome (DIHS)
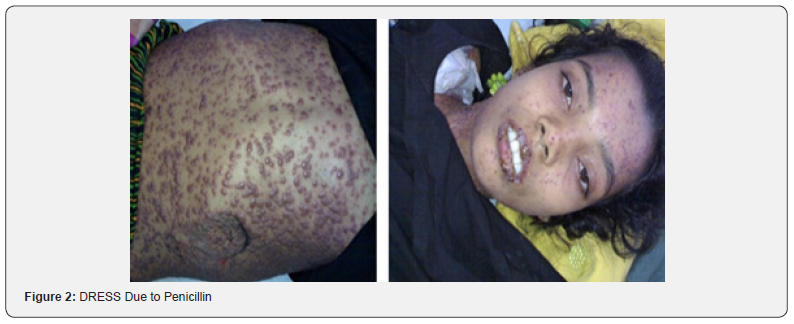
DIHS is also known as drug reaction with eosinophilia and systemic symptoms (DRESS), hypersensitivity syndrome (HSS), drug-induced delayed multiorgan hypersensitivity syndrome (DIDMOHS). It is an idiosyncratic, severe cutaneous adverse drug reaction (ScADR) (Choudhary, McLeod, Torchia, & Romanelli, 2013). Characterized by a triad of fever, skin eruption and systemic organ involvement. Systemic signs like lymphadenopathy (50% cases), hematological abnormalities, and organ damage like hepatitis (75-90% cases) may also be seen. Symptoms appear between 2-6 or 9 weeks after drug exposure. Skin eruptions follow high fever (38°C-40°C) in almost 100% of cases. Concomitant viral infection with viruses like herpes virus, CMV, EBV and ethnic predispositions are proposed. Drugs like sulfonamides, fluoroquinolones and minocycline or their metabolites may cause this rare but life-threatening syndrome. Following a high fever, there is an appearance of pruritic exanthem, which is a diffuse, erythematous macular or maculopapular rash. It may be accompanied by facial edema. Occasionally, pustules, blisters, lichenoid, eczematous, and exfoliative skin lesions are visible (Figure 2). Besides internal organ damage, there could be palpable lymph nodes present. The liver, kidney, and lungs are the most affected systemic organs. The heart, gastrointestinal, and central nervous systems may also show evidence of damage, though less commonly. On laboratory examination, leukocytosis, eosinophilia, atypical lymphocytes, thrombocytopenia, and abnormal liver function test (LFT) and renal function test (RFT) may be found. If mucosal involvement is at all present, it is less severe than SJS/TEN. DRESS brings high risk of developing autoimmune diseases during recovery thus requiring long-term monitoring [12, 17, 41-43]. The pathogenesis of DRESS is not well understood. There may be a genetic deficiency of drug metabolizing enzymes, which leads to an accumulation of drug metabolites that bind covalently to macromolecules in the cells causing cell death. Activated eosinophils, as well-activated inflammatory cascade induced by interleukin-5 by drug-specific T-cells, may also contribute. Genetic associations like HLA-B 1502 in carbamazepine.
Drug-Induced Erythroderma or Exfoliative Dermatitis (ED)
A rare and severe skin disorder that occurs due to various causes like- viral infections, previous dermatoses (like- psoriasis and eczema), drug reactions, malignancies, and idiopathic disorders. There is extreme skin irritation, diffuse erythema, and desquamation of skin on almost whole or most body surfaces (>90%). There is pruritus, scaling, fever, lymphadenopathy, and visceral enlargement. Occasionally, edema, hyperkeratosis and mucosal involvement may be present [40, 45-47]. Histopathology shows hyperkeratosis, parakeratosis, acanthosis, and chronic inflammatory infiltrates surrounding blood vessels with eosinophils [40]. Drugs like- antivirals, antibiotics, carbamazepine, thalidomide [46] and NSAIDs [48] may cause ED. Among antimicrobials, common culprits are sulfonamides, antimalarials, penicillins, isoniazid, thioacetazone, and streptomycin [40, 48].
Contact Dermatitis (CD)
This immune-mediated delayed allergic skin reaction [49] appears within 48 to 72 hours drug re-exposure. The rash is vesicular and pruritic (may be intense) with red spots at points of contact, occasionally spread beyond and having visible borders. These may lead to erythema and scaling. There is a dramatic flaring with erythema, vesicles, and bullae in acute cases; chronic cases may make the skin thickened with cracks and fissures. Common triggering agents include poison ivy, nickel, oxybenzone, neomycin, and topical benzocaine [50]. The pathophysiology involves T cell-mediated responses to such antigens that involve CD4+ and CD8+ alphabeta+ T lymphocytes, as well CD4 /CD8 gamma delta+ T cells. Other immune cells may also be involved [51].
Leukocytoclastic Vasculitis (LcV)
It is a small-vessel vasculitis of the dermal capillaries and venules. May be of idiopathic origin (up to 50%) or caused by infections, neoplasms, autoimmune disorders, and drugs [52]. It is the commonest of all vasculitic skin lesions [53]. There is palpable purpura on the lower limbs and small vessel involvement. About 30% of the patients have extracutaneous involvement. Antibiotics like beta-lactams, erythromycin, clindamycin, vancomycin, and sulfonamides are common offenders [52]. As well imipenem-dilatating and levofloxacin-induced LcV have been reported [54-55]. Lesions appear after 1-3 weeks of initiation of the offending drug [52]. The pathophysiology includes immune complex deposition in small vessel walls. Besides activation of the complement system, there is the recruitment of neutrophils, leading to injury to vessel walls. Secondary erythrocyte exudation, fibrin, and serum also do take place. Lysosomal enzymes like collagenases and elastases, and reactive oxygen species may lead to fibrinoid necrosis of small vessel walls. Lymphokines support the evolution of the process, as well IL-1, IL-6, IL-8, and TNF are increased in circulation [52].
Lesions present as erythematous macules with palpable purpura on both sides of the dependent body parts, like the lower limbs and buttocks. Rarely unilateral or localized lesions are seen. Hemorrhagic vesicles and bulla, pustules, nodules, and crusted ulcers may be revealed too. The size of the lesions varies from 1 mm to 1 cm. May appear at once in crops or in cycles showing lesions with different stages [52].
- Research article
- Abstract
- Background and Introduction
- Method
- Review
- Type IV hypersensitivity
- Urticarial cADRs
- Serum Sickness-Like Reaction
- Bullous Eruptions
- Drug-Induced Pemphigus (DIP)
- Drug Induced Linear IgA Bullous Dermatosis (LABD)
- Stevens-Johnson Syndrome (SJS) and Toxic Epidermal Necrolysis (TEN)
- Pustular cADRs
- Acute Generalized Exanthematous Pustulosis (AGEP)
- Acute Localized Exanthematous Pustulosis (ALEP)
- Drug Induced Sub Corneal Pustular Dermatosis (SPD)
- Miscellaneous
- Drug Induced Alopecia
- Drug-Induced Photosensitivity
- Drug-Induced Vasculitis
- Severe Cutaneous Adverse Drug Reactions (ScADRs)
- Cutaneous Adverse Drug Reactions to Some Common Antibiotics
- Discussion
- Conclusion
- References
Urticarial cADRs
Urticaria
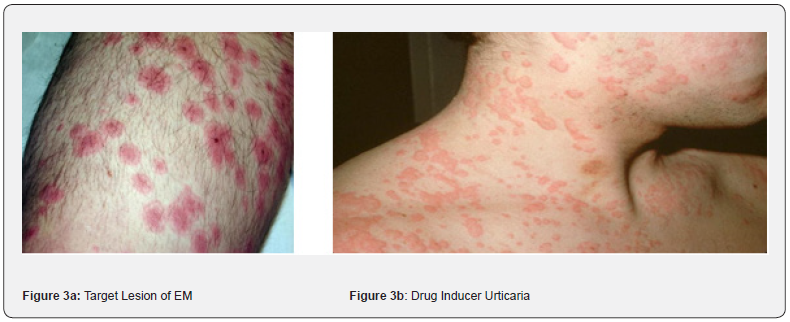
It is the second most common type of cADRs. Presents as itchy wheals (hives) on any part of the body, which are usually erythematous (Figure 3b). The lesions may or may not accompany angioedema [41] (Walsh, 2014). Disappears within 24 hours without any marks on the skin. The hives show a triad of signs: a central swelling surrounded by erythema, an itching or burning sensation, and transitory nature. The pathophysiology involves release of inflammatory mediators from mast cells and basophils due to immune mechanism like Type-I hypersensitivity (Ig E mediated) or a non-immune mechanism like direct degranulation of immune cells by drugs like NSAIDs. Initially, single, or multiple urticarial lesions of varying sizes may appear, which may be small pink papules, macules or larger red wheals [12,17,40]. Histologically, dermal edema with neutrophil, lymphocyte, and eosinophil infiltrates. Edema of subcutaneous tissue is called angioedema. Angioedema and urticaria coexist in 50% of cases. Angioedema presents as pinkish or pale swelling of the skin where the skin is loose, like- the face, genital area, and mucosa involving the mouth, larynx, and trachea. Anaphylaxis is an immediate Type-I hypersensitivity reaction, which can lead to respiratory collapse, shock, and death. Multiorgan involvement in anaphylaxis leads to hypotension, bronchospasm, and GIT symptoms. Culprit drugs are antibiotics like- penicillin, cephalosporins, tetracyclines, and sulfonamides besides other drugs like- general anesthetics, phenytoin, morphine, codeine, NSAIDs, ACE inhibitors [12, 17-18, 40-42].
Serum Sickness
A Type-III hypersensitivity reaction initiated by immune complexes and characterized by fever, rash, and joint involvement. Appears after 1-2 weeks of exposure to drugs like immune-modulating agents, e.g., rabies vaccine, antivenoms etc. The rash is urticarial and may present as maculopapular or vascular eruptions. There is arthritis in hands, feet, ankles, knees, and shoulder joints. Besides, there could be accompanying clinical features like- lymphadenopathy, neuropathy, vasculitis, and headache. Being a self-limiting condition, it usually resolves within several weeks after the drug causing it is stopped [17].
- Research article
- Abstract
- Background and Introduction
- Method
- Review
- Type IV hypersensitivity
- Urticarial cADRs
- Serum Sickness-Like Reaction
- Bullous Eruptions
- Drug-Induced Pemphigus (DIP)
- Drug Induced Linear IgA Bullous Dermatosis (LABD)
- Stevens-Johnson Syndrome (SJS) and Toxic Epidermal Necrolysis (TEN)
- Pustular cADRs
- Acute Generalized Exanthematous Pustulosis (AGEP)
- Acute Localized Exanthematous Pustulosis (ALEP)
- Drug Induced Sub Corneal Pustular Dermatosis (SPD)
- Miscellaneous
- Drug Induced Alopecia
- Drug-Induced Photosensitivity
- Drug-Induced Vasculitis
- Severe Cutaneous Adverse Drug Reactions (ScADRs)
- Cutaneous Adverse Drug Reactions to Some Common Antibiotics
- Discussion
- Conclusion
- References
Serum Sickness-Like Reaction
An immunological skin reaction to drugs due to antigen-antibody (Ag-Ab) complex formation, characterized by the sudden appearance of rash with joint inflammation with or without fever. Ag-Ab complex induces Type-IV hypersensitivity reaction. It is dissimilar to serum sickness in many ways. There is an urticaria-like skin rash characterized by erythematous papules and macules of varying sizes which are not transient and may persist for several days. Eye and lip edema may be present without respiratory symptoms or anaphylaxis. Causative agents are- β-lactams like amoxicillin and cephalosporins [17].
- Research article
- Abstract
- Background and Introduction
- Method
- Review
- Type IV hypersensitivity
- Urticarial cADRs
- Serum Sickness-Like Reaction
- Bullous Eruptions
- Drug-Induced Pemphigus (DIP)
- Drug Induced Linear IgA Bullous Dermatosis (LABD)
- Stevens-Johnson Syndrome (SJS) and Toxic Epidermal Necrolysis (TEN)
- Pustular cADRs
- Acute Generalized Exanthematous Pustulosis (AGEP)
- Acute Localized Exanthematous Pustulosis (ALEP)
- Drug Induced Sub Corneal Pustular Dermatosis (SPD)
- Miscellaneous
- Drug Induced Alopecia
- Drug-Induced Photosensitivity
- Drug-Induced Vasculitis
- Severe Cutaneous Adverse Drug Reactions (ScADRs)
- Cutaneous Adverse Drug Reactions to Some Common Antibiotics
- Discussion
- Conclusion
- References
Bullous Eruptions
Bullous Eruptions
The Bullous drug reactions are characterized by fluid-filled blisters or bullae on the skin and mucous membranes. They are particularly challenging as most of these are the most severe cADRs [56-57]. Bullous eruptions include different morphological types, namely- fixed drug eruption, erythema multiforme, drug-induced bullous pemphigoid, drug-induced pemphigus, drug-induced linear IgA bullous dermatosis, drug-induced dermatitis herpitiformis, Stevens-Johnson syndrome and toxic epidermal necrolysis, bullous DRESS, contact dermatitis [17,56].
Fixed Drug Eruption (FDE)
A common type of cADR characterized by one or more round to oval erythematous macules, patches, or plaques with or without overlying blistering. There is the preference for face, lips, genitalia, buttock, and upper extremities, but it may occur anywhere. The lesions appear 24 hours to several days after the offending drug exposure. The eruptions tend to recur with repeated drug exposure in the same location, hence the name FDE. Common drugs causing FDE are- NSAIDs, paracetamol, antihistamines, and antimicrobials. Generalized FDE mimics TEN [12,17-18,41].
Erythema Multiforme (EM)
Also known as erythema nodosum (EN). The characteristic feature of EM is targeting lesions, with a darker centre and paler periphery having concentric erythematous rings (Figure 3a). Two types- EM minor and EM major. The major type shows mucosal involvement. Commonly caused by viral infections like herpes simplex virus, but also may be caused by drugs like- sulfonamides and penicillin Abs [12,41].
Drug-Induced Bullous Pemphigoid (DIBP)
It is an autoimmune condition with patches and tense clear blistering on the affected skin that do not break easily. The bullae may overly erythematous or urticarial plaques. Sudden onset may look like EM and rarely involves mucous membrane. ABs, analgesics, frusemide, captopril, penicillamine, dipeptidyl peptidase-4 inhibitors (gliptins), and sulfasalazine are common agents causing DIBP. This is common among young adults in contrast to the idiopathic type, which is common in older adults. The mechanism is not fully understood yet, but there is a genetic predisposition (specific HLA alles involved) [17,57].
- Research article
- Abstract
- Background and Introduction
- Method
- Review
- Type IV hypersensitivity
- Urticarial cADRs
- Serum Sickness-Like Reaction
- Bullous Eruptions
- Drug-Induced Pemphigus (DIP)
- Drug Induced Linear IgA Bullous Dermatosis (LABD)
- Stevens-Johnson Syndrome (SJS) and Toxic Epidermal Necrolysis (TEN)
- Pustular cADRs
- Acute Generalized Exanthematous Pustulosis (AGEP)
- Acute Localized Exanthematous Pustulosis (ALEP)
- Drug Induced Sub Corneal Pustular Dermatosis (SPD)
- Miscellaneous
- Drug Induced Alopecia
- Drug-Induced Photosensitivity
- Drug-Induced Vasculitis
- Severe Cutaneous Adverse Drug Reactions (ScADRs)
- Cutaneous Adverse Drug Reactions to Some Common Antibiotics
- Discussion
- Conclusion
- References
Drug-Induced Pemphigus (DIP)
Characterised by flaccid blisters which break easily and often only erosions and/or crusting is visible. Nikolsky’s sign (detachment of the epidermis from the dermis by a shearing force) [18] may be positive. Mucous membranes may be involved in 10–15% of cases. The pathophysiology involves the formation of autoantibodies reacting with desmogleins. ABs like penicillin, cephalosporins and vancomycin cause DIP. Blistering resolves after discontinuation of the drugs, but rarely may persist like non-drug induced pemphigus [12,57].
- Research article
- Abstract
- Background and Introduction
- Method
- Review
- Type IV hypersensitivity
- Urticarial cADRs
- Serum Sickness-Like Reaction
- Bullous Eruptions
- Drug-Induced Pemphigus (DIP)
- Drug Induced Linear IgA Bullous Dermatosis (LABD)
- Stevens-Johnson Syndrome (SJS) and Toxic Epidermal Necrolysis (TEN)
- Pustular cADRs
- Acute Generalized Exanthematous Pustulosis (AGEP)
- Acute Localized Exanthematous Pustulosis (ALEP)
- Drug Induced Sub Corneal Pustular Dermatosis (SPD)
- Miscellaneous
- Drug Induced Alopecia
- Drug-Induced Photosensitivity
- Drug-Induced Vasculitis
- Severe Cutaneous Adverse Drug Reactions (ScADRs)
- Cutaneous Adverse Drug Reactions to Some Common Antibiotics
- Discussion
- Conclusion
- References
Drug Induced Linear IgA Bullous Dermatosis (LABD)
LABD resembles the idiopathic variety. Presents with tense small and large blisters, often in ring-like arrangements on the trunk, arms, and legs. It may also involve the palms and soles, but rarely mucous membranes. It may become more severe and resemble TEN. Histopathological examination usually does not show circulating IgA autoantibodies to the basement membrane zone. Lesions appear 1–2 weeks after starting the drug and disappear in 1-3 days. Rechallenge with the drug causes a more severe reaction with a shorter latency time and longer clearance time. Vancomycin is the major culprit, but other drugs may also cause LABD. Suggested mechanism is- drug activating CD8+ T cells, which release Interleukin (IL)-5, it then influences IgA expression. The IgA antibody then targets numerous antigens [57].
- Research article
- Abstract
- Background and Introduction
- Method
- Review
- Type IV hypersensitivity
- Urticarial cADRs
- Serum Sickness-Like Reaction
- Bullous Eruptions
- Drug-Induced Pemphigus (DIP)
- Drug Induced Linear IgA Bullous Dermatosis (LABD)
- Stevens-Johnson Syndrome (SJS) and Toxic Epidermal Necrolysis (TEN)
- Pustular cADRs
- Acute Generalized Exanthematous Pustulosis (AGEP)
- Acute Localized Exanthematous Pustulosis (ALEP)
- Drug Induced Sub Corneal Pustular Dermatosis (SPD)
- Miscellaneous
- Drug Induced Alopecia
- Drug-Induced Photosensitivity
- Drug-Induced Vasculitis
- Severe Cutaneous Adverse Drug Reactions (ScADRs)
- Cutaneous Adverse Drug Reactions to Some Common Antibiotics
- Discussion
- Conclusion
- References
Stevens-Johnson Syndrome (SJS) and Toxic Epidermal Necrolysis (TEN)
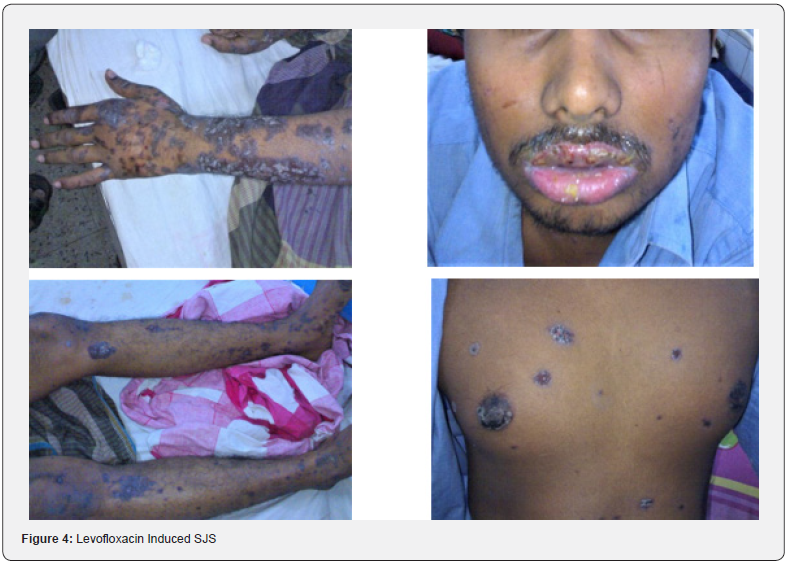
Also known as Lyell Syndrome [40]. These are very rare but fatal cADRs, considered among the severe cADR class because of high morbidity, mortality, and long-term consequences. The incidence of SJS is 1-2 cases per million person-years, and TEN is 0.1-1.2 cases per million person-years [40]. SJS and TEN are considered to be two spectrums of the same condition depending on the percentage of skin detachment. In SJS <10% of the body surface area (BSA) is involved, whereas TEN involves >30% BSA. SJS/TEN overlap is a condition placed in between these two spectra and involves between 10-30% of BSA. There is widespread keratinocyte death in the epidermis leading to loss of the epidermis with mucosal erosions [40-41].
SJS/TEN develop 7-21 days of drug exposure. Present as painful, erythematous, target-like dusky or purpuric macules and papules, which progress to flaccid blisters rapidly. In about 95% of cases, hemorrhagic erosions [40] may appear, and Nikolsky’s sign is positive. Besides, patients present with nonspecific symptoms like- high fever, respiratory problems, and severe malaise. The lesions start appearing on the face and/or thorax and then spread symmetrically to involve the rest of the body. Mucous membranes in the mouth, conjunctiva, genital and anal region may show lesions before cutaneous lesions appear. In severe form, internal organs like kidneys, liver, lungs, GIT, bone marrow and joints may be affected [17]. About 10% patients with SJS may die, whereas this may reach up to 40% with TEN. Sulfonamides, penicillins, fluroquinolones are common ABs that are implicated as the cause (Figure 4) [12, 41]. Other drugs like- anticonvulsants, NSAIDs (oxicam), allopurinol, and nevirapine are associated with high risk. SJS/TEN is dose-independent ADR [40]. The mechanism is T cell-mediated immune reaction. Drugs or its toxic metabolites working as haptens provide antigenic stimulus. This initiated a delayed hypersensitivity reaction. Drug-specific CD4+ T cells are found in the dermis and CD8+ cells in the epidermis. The massive release of cytokines and soluble factors like perforin, granzyme B, and granulysin from the immune cells cause widespread apoptosis, especially of the keratinocytes. Granulysin released from cytotoxic T lymphocytes and Natural Killer (NK) cells causes damage to mitochondria and, ultimately, apoptosis. Genetic factors and some HLA alleles may be involved in the pathophysiology of this spectrum [40].
- Research article
- Abstract
- Background and Introduction
- Method
- Review
- Type IV hypersensitivity
- Urticarial cADRs
- Serum Sickness-Like Reaction
- Bullous Eruptions
- Drug-Induced Pemphigus (DIP)
- Drug Induced Linear IgA Bullous Dermatosis (LABD)
- Stevens-Johnson Syndrome (SJS) and Toxic Epidermal Necrolysis (TEN)
- Pustular cADRs
- Acute Generalized Exanthematous Pustulosis (AGEP)
- Acute Localized Exanthematous Pustulosis (ALEP)
- Drug Induced Sub Corneal Pustular Dermatosis (SPD)
- Miscellaneous
- Drug Induced Alopecia
- Drug-Induced Photosensitivity
- Drug-Induced Vasculitis
- Severe Cutaneous Adverse Drug Reactions (ScADRs)
- Cutaneous Adverse Drug Reactions to Some Common Antibiotics
- Discussion
- Conclusion
- References
Pustular cADRs
Drug-Induced Palmoplantar Pustulosis:
Psoriasiform drug eruption is a drug-induced chronic, debilitating, immune-mediated, inflammatory skin condition with a prevalence of 0.65 to 4.8%. Clinically and histopathologically resembles psoriasis. There is cellular infiltration, papillomatosis, and epidermal hyperplasia with elongation of rete ridges. Hyper-granulosis and parakeratosis may also be found (Kim & Rosso, 2010). Drugs like beta-blockers, lithium, tetracyclines, NSAIDs, adalimumab, and synthetic antimalarials may cause Palmoplantar pustular psoriasis [58-59]. Drug-induced psoriasis and drug-aggravated psoriasis are two variants. Former abates on stoppage of the offending drug but the next one continues to progress [60].
- Research article
- Abstract
- Background and Introduction
- Method
- Review
- Type IV hypersensitivity
- Urticarial cADRs
- Serum Sickness-Like Reaction
- Bullous Eruptions
- Drug-Induced Pemphigus (DIP)
- Drug Induced Linear IgA Bullous Dermatosis (LABD)
- Stevens-Johnson Syndrome (SJS) and Toxic Epidermal Necrolysis (TEN)
- Pustular cADRs
- Acute Generalized Exanthematous Pustulosis (AGEP)
- Acute Localized Exanthematous Pustulosis (ALEP)
- Drug Induced Sub Corneal Pustular Dermatosis (SPD)
- Miscellaneous
- Drug Induced Alopecia
- Drug-Induced Photosensitivity
- Drug-Induced Vasculitis
- Severe Cutaneous Adverse Drug Reactions (ScADRs)
- Cutaneous Adverse Drug Reactions to Some Common Antibiotics
- Discussion
- Conclusion
- References
Acute Generalized Exanthematous Pustulosis (AGEP)
AGEP is a rare skin drug reaction with sterile, non-follicular, superficial, small pustules on an erythematous base accompanied by fever, itching, burning, and occasional systematic symptoms. The incidence is 1-5 patients per million per year. At the onset, there is edematous erythema on the affected skin, usually on the armpits, neck, and groin (intertriginous regions) then spreading to the trunk and limbs. Facial edema, purpura, vesicles, and EM-like lesions may be visible too. Pustules are followed by desquamation. Rare mucosal involvement (up to 20%), mostly oral. Up to 90% of AGEP is due to drug exposure, and the lesions appear within 1-2 days of offending drug exposure. There is peripheral neutrophilia and leukocytosis in 90% of patients [12, 17, 40-41]. Offending drugs may include antibiotics (Walsh, 2014) like aminopenicillins (ampicillin, amoxycillin), pristinamycin, quinolones, sulfonamides, tetracyclines, and other drugs like terbinafine, ketoconazole, fluconazole, diltiazem, and hydroxychloroquine [12, 17, 40].
The lesions show spontaneous resolution on discontinuation of the drug within two weeks followed by superficial desquamation. Pathogenesis is unclear, but AGEP is a type IV hypersensitivity reaction. It is suggested that there is activation, expansion, and migration of drug-specific drug CD4 and CD8 T cells to the site of reaction. This leads to the apoptosis of keratinocytes and the formation of vesicles. Neutrophils play a significant role, especially in adaptative immune reactions. CD4+ helper T cells and CD8+ cytotoxic T cells produce cytokines, IL-17, IL-22, GCSF and TNF-α. These are involved in the recruitment of neutrophils [40].
- Research article
- Abstract
- Background and Introduction
- Method
- Review
- Type IV hypersensitivity
- Urticarial cADRs
- Serum Sickness-Like Reaction
- Bullous Eruptions
- Drug-Induced Pemphigus (DIP)
- Drug Induced Linear IgA Bullous Dermatosis (LABD)
- Stevens-Johnson Syndrome (SJS) and Toxic Epidermal Necrolysis (TEN)
- Pustular cADRs
- Acute Generalized Exanthematous Pustulosis (AGEP)
- Acute Localized Exanthematous Pustulosis (ALEP)
- Drug Induced Sub Corneal Pustular Dermatosis (SPD)
- Miscellaneous
- Drug Induced Alopecia
- Drug-Induced Photosensitivity
- Drug-Induced Vasculitis
- Severe Cutaneous Adverse Drug Reactions (ScADRs)
- Cutaneous Adverse Drug Reactions to Some Common Antibiotics
- Discussion
- Conclusion
- References
Acute Localized Exanthematous Pustulosis (ALEP)
It is a localized form of AGEP. There are multiple non-follicular sterile pustular lesions on the background of an erythematous rash. Appears suddenly after drug exposure, affecting the face, neck, or chest. Like the onset, remission is also quick, within days even in the absence of treatment. There may accompany fever and neutrophilic leukocytosis without systemic organ involvement [40].
- Research article
- Abstract
- Background and Introduction
- Method
- Review
- Type IV hypersensitivity
- Urticarial cADRs
- Serum Sickness-Like Reaction
- Bullous Eruptions
- Drug-Induced Pemphigus (DIP)
- Drug Induced Linear IgA Bullous Dermatosis (LABD)
- Stevens-Johnson Syndrome (SJS) and Toxic Epidermal Necrolysis (TEN)
- Pustular cADRs
- Acute Generalized Exanthematous Pustulosis (AGEP)
- Acute Localized Exanthematous Pustulosis (ALEP)
- Drug Induced Sub Corneal Pustular Dermatosis (SPD)
- Miscellaneous
- Drug Induced Alopecia
- Drug-Induced Photosensitivity
- Drug-Induced Vasculitis
- Severe Cutaneous Adverse Drug Reactions (ScADRs)
- Cutaneous Adverse Drug Reactions to Some Common Antibiotics
- Discussion
- Conclusion
- References
Drug Induced Sub Corneal Pustular Dermatosis (SPD)
Also known as Sneddon-Wilkinson disease as it was described by Sneddon and Wilkinson in 1956 for the first time. SPD is a rare, chronic, relapsing pustular skin reaction where the eruptions constitute subcorneal pustules containing neutrophils. The lesions present as discrete, flaccid pustules or grouped vesicles on the flexor surfaces [61]. Etiology is unknown [62].
- Research article
- Abstract
- Background and Introduction
- Method
- Review
- Type IV hypersensitivity
- Urticarial cADRs
- Serum Sickness-Like Reaction
- Bullous Eruptions
- Drug-Induced Pemphigus (DIP)
- Drug Induced Linear IgA Bullous Dermatosis (LABD)
- Stevens-Johnson Syndrome (SJS) and Toxic Epidermal Necrolysis (TEN)
- Pustular cADRs
- Acute Generalized Exanthematous Pustulosis (AGEP)
- Acute Localized Exanthematous Pustulosis (ALEP)
- Drug Induced Sub Corneal Pustular Dermatosis (SPD)
- Miscellaneous
- Drug Induced Alopecia
- Drug-Induced Photosensitivity
- Drug-Induced Vasculitis
- Severe Cutaneous Adverse Drug Reactions (ScADRs)
- Cutaneous Adverse Drug Reactions to Some Common Antibiotics
- Discussion
- Conclusion
- References
Miscellaneous
Drug Induced Lupus (DI-SLE)
Exposure to certain drugs may lead to systemic lupus erythematosus (SLE) like symptoms or DI-SLE, which is an autoimmune reaction. A genetically susceptible person develops DI-SLE on exposure to environmental triggers. In 1954 hydralazine was described to cause lupus-like symptoms. More than 100 offending agents have been implicated, like procainamide, minocycline, isoniazid, quinidine, methyldopa, interferon-alpha, rifampin, phenytoin, penicillamine, chlorpromazine, carbamazepine, ethosuximide, propylthiouracil, and sulfasalazine. Statins, antiarrhythmic medications, ACE inhibitors, proton pump inhibitors, gold salts, NSAIDs, and oral contraceptives are also among the suspected culprits. Of all lupus cases, 6% to 12% are DI-SLE, and the annual incidence is 15,000 to 30,000 new cases per year in the US. Anti-histone Abs may be found (in 75%), though anti-dsDNA Abs are less common to be found. Patients present with fever, arthralgia/arthritis, and myalgia like symptoms with rare involvement of internal organs like kidneys and CNS. Skin presentation includes annular-polycyclic or psoriasis-like lesions on photosensitive areas accompanying anti-SSA Abs and ANAs. Symptoms abate soon after discontinuing the drug causing DI-SLE [17, 63].
- Research article
- Abstract
- Background and Introduction
- Method
- Review
- Type IV hypersensitivity
- Urticarial cADRs
- Serum Sickness-Like Reaction
- Bullous Eruptions
- Drug-Induced Pemphigus (DIP)
- Drug Induced Linear IgA Bullous Dermatosis (LABD)
- Stevens-Johnson Syndrome (SJS) and Toxic Epidermal Necrolysis (TEN)
- Pustular cADRs
- Acute Generalized Exanthematous Pustulosis (AGEP)
- Acute Localized Exanthematous Pustulosis (ALEP)
- Drug Induced Sub Corneal Pustular Dermatosis (SPD)
- Miscellaneous
- Drug Induced Alopecia
- Drug-Induced Photosensitivity
- Drug-Induced Vasculitis
- Severe Cutaneous Adverse Drug Reactions (ScADRs)
- Cutaneous Adverse Drug Reactions to Some Common Antibiotics
- Discussion
- Conclusion
- References
Drug Induced Alopecia
Alopecia means loss of hair on the scalp or on the body [64]. Drug induced alopecia is mostly a reversible condition, without any scarring, where there could be diffuse loss of hair on the introduction of a new drug. Commonly caused by chemotherapeutic agents. Heparin, warfarin, ACEIs, beta-blockers, valproic acid, carbamazepine, phenytoin, lithium, cimetidine, retinoids, antithyroid drugs, cholesterol-lowering drugs, interferons, anti-infective agents, amphetamines, NSAIDs, bromocriptine, levodopa, some antipsychotics, and anti-anxiety drugs may also cause hair loss. Either there is a falling of actively growing hairs (anagen effluvium) due to abrupt cessation of mitotic activity in rapidly dividing hair matrix cells or shedding of resting or bulb hairs (telogen effluvium) due to precipitating the follicles into premature rest [65-67].
- Research article
- Abstract
- Background and Introduction
- Method
- Review
- Type IV hypersensitivity
- Urticarial cADRs
- Serum Sickness-Like Reaction
- Bullous Eruptions
- Drug-Induced Pemphigus (DIP)
- Drug Induced Linear IgA Bullous Dermatosis (LABD)
- Stevens-Johnson Syndrome (SJS) and Toxic Epidermal Necrolysis (TEN)
- Pustular cADRs
- Acute Generalized Exanthematous Pustulosis (AGEP)
- Acute Localized Exanthematous Pustulosis (ALEP)
- Drug Induced Sub Corneal Pustular Dermatosis (SPD)
- Miscellaneous
- Drug Induced Alopecia
- Drug-Induced Photosensitivity
- Drug-Induced Vasculitis
- Severe Cutaneous Adverse Drug Reactions (ScADRs)
- Cutaneous Adverse Drug Reactions to Some Common Antibiotics
- Discussion
- Conclusion
- References
Drug-Induced Photosensitivity
An interaction between the drug or its active metabolite and sun exposure gives rise to this type of reaction. Two main mechanisms have been described: (1) photoallergic and (2) phototoxic reactions. The former type includes sensitization by the drug towards T-cell-mediated or antibody-mediated response. The sensitizing drug/metabolite binds to certain proteins to form photo-antigens. A little amount of the drug may suffice to induce the reactions. There is a latent time of several days after the exposure. Drugs like thiazide diuretics, NSAIDs, simvastatin, itraconazole, griseofulvin, other antifungals, antimalarials, beta-blockers, escitalopram, isotretinoin, TNF inhibitors, and antibiotics like tetracyclines, cefotaxime, fluoroquinolones, and sulfonamides may cause these reactions. Minocycline may rarely cause SLE-like photoallergic reactions as well as isoniazid and ethambutol may lead to photoallergic rash like pellagra. The lesions may be eczematous eruptions or lichenoid reactions [12, 17]. Phototoxic reactions have different mechanisms and are the results of direct cellular damage by the photoactive agents. More common than the former type and develop very quickly (within hours) of drug exposure. It is dose dependent reaction and shows no cross-reactivity between similar agents. There is erythema on the skin, looking like exaggerated sunburn. There may be hyperpigmentation, onycholysis, pseudo-porphyria and telangiectasis [12, 17].
- Research article
- Abstract
- Background and Introduction
- Method
- Review
- Type IV hypersensitivity
- Urticarial cADRs
- Serum Sickness-Like Reaction
- Bullous Eruptions
- Drug-Induced Pemphigus (DIP)
- Drug Induced Linear IgA Bullous Dermatosis (LABD)
- Stevens-Johnson Syndrome (SJS) and Toxic Epidermal Necrolysis (TEN)
- Pustular cADRs
- Acute Generalized Exanthematous Pustulosis (AGEP)
- Acute Localized Exanthematous Pustulosis (ALEP)
- Drug Induced Sub Corneal Pustular Dermatosis (SPD)
- Miscellaneous
- Drug Induced Alopecia
- Drug-Induced Photosensitivity
- Drug-Induced Vasculitis
- Severe Cutaneous Adverse Drug Reactions (ScADRs)
- Cutaneous Adverse Drug Reactions to Some Common Antibiotics
- Discussion
- Conclusion
- References
Drug-Induced Vasculitis
Presents with palpable purpura of varying sizes, petechiae, papules, and plaques and progressing to pustules or bullae. The bullae may ultimately evolve into ulcerative-necrotic lesions and heal, leaving hyperpigmented areas. Drugs working as antigens may induce inflammatory damage to the blood vessel walls in the skin. There might be underlying diseases like connective tissue disease, inflammatory bowel disease or other malignancies. Cutaneous small vessel vasculitis (CSVV) also known as leukocytoclastic vasculitis, affects post-capillary skin venules. Signs of vasculitis appear between 7-21 days after drug exposure. Lesions often appear on the areas of stasis, like on the lower legs, but may involve ankles, lower trunk, and upper limbs. A patient may experience burning pain and/or itching. Systemic symptoms include fever, arthralgia, myalgia, and anorexia with rare renal or other organ involvement. In CSVV, there is fibrinoid necrosis of vessel walls with upper dermal perivascular infiltration by neutrophils. Eosinophils may also be found in the infiltrates. IgM and/or complement C3 deposits on vessel walls is seen in 80% of cases [40]. Drugs causing vasculitis are antibiotics, diuretics, NSAIDs, anticonvulsants, antipsychotics, TNF-α inhibitors, rituximab, and INT-β. The proposed mechanism suggests that complement activation causes the drug-induced immune complex deposits in the vessel walls, stimulating proinflammatory cytokines, chemokines, and vasoactive amines. All these lead to neutrophil recruitment, degranulation of immune cells releasing collagenase and elastase enzymes, besides generating reactive oxygen species. The result is inflammation and fibrinoid necrosis of vessel walls [40].
- Research article
- Abstract
- Background and Introduction
- Method
- Review
- Type IV hypersensitivity
- Urticarial cADRs
- Serum Sickness-Like Reaction
- Bullous Eruptions
- Drug-Induced Pemphigus (DIP)
- Drug Induced Linear IgA Bullous Dermatosis (LABD)
- Stevens-Johnson Syndrome (SJS) and Toxic Epidermal Necrolysis (TEN)
- Pustular cADRs
- Acute Generalized Exanthematous Pustulosis (AGEP)
- Acute Localized Exanthematous Pustulosis (ALEP)
- Drug Induced Sub Corneal Pustular Dermatosis (SPD)
- Miscellaneous
- Drug Induced Alopecia
- Drug-Induced Photosensitivity
- Drug-Induced Vasculitis
- Severe Cutaneous Adverse Drug Reactions (ScADRs)
- Cutaneous Adverse Drug Reactions to Some Common Antibiotics
- Discussion
- Conclusion
- References
Severe Cutaneous Adverse Drug Reactions (ScADRs)
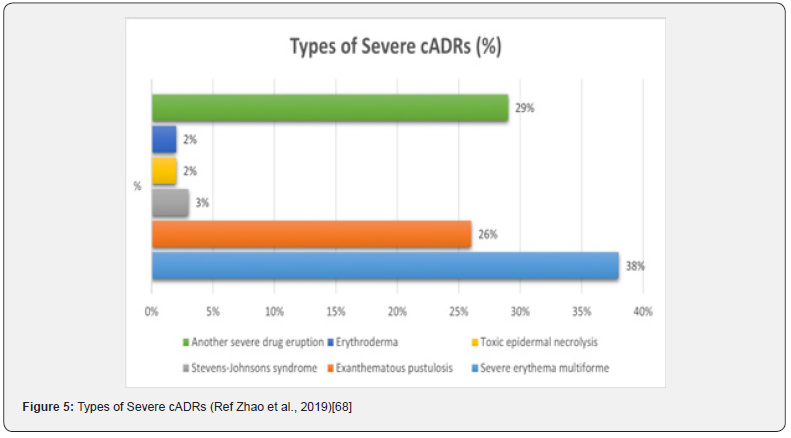
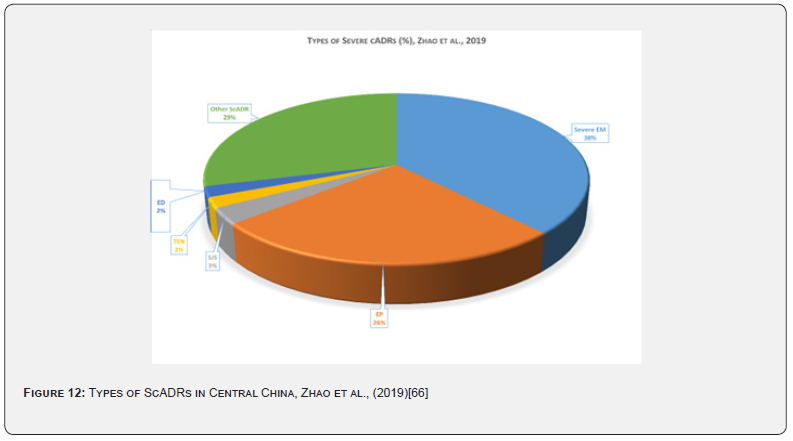
Another way of categorizing cADRs is by dividing them into non-severe cADRs and severe cADRs or ScADRs. Severe cADRs are a group of potentially lethal ADRs involving the skin, mucous membranes, and internal viscera. This may lead to pronounced disability, needing hospitalization, intensive care, and specialized care. ScADRs include SJS, TEN, SJS/TEN overlap, DRESS, AGEP, GBFDE, and drug-induced acute ED. Various types of severe cADRs were described by Zhao et al., (2019) (Figure 5) [68].
- Research article
- Abstract
- Background and Introduction
- Method
- Review
- Type IV hypersensitivity
- Urticarial cADRs
- Serum Sickness-Like Reaction
- Bullous Eruptions
- Drug-Induced Pemphigus (DIP)
- Drug Induced Linear IgA Bullous Dermatosis (LABD)
- Stevens-Johnson Syndrome (SJS) and Toxic Epidermal Necrolysis (TEN)
- Pustular cADRs
- Acute Generalized Exanthematous Pustulosis (AGEP)
- Acute Localized Exanthematous Pustulosis (ALEP)
- Drug Induced Sub Corneal Pustular Dermatosis (SPD)
- Miscellaneous
- Drug Induced Alopecia
- Drug-Induced Photosensitivity
- Drug-Induced Vasculitis
- Severe Cutaneous Adverse Drug Reactions (ScADRs)
- Cutaneous Adverse Drug Reactions to Some Common Antibiotics
- Discussion
- Conclusion
- References
Cutaneous Adverse Drug Reactions to Some Common Antibiotics
The following table shows the common cADRs caused by some commonly used antibiotics (Table 1) [69].
- Research article
- Abstract
- Background and Introduction
- Method
- Review
- Type IV hypersensitivity
- Urticarial cADRs
- Serum Sickness-Like Reaction
- Bullous Eruptions
- Drug-Induced Pemphigus (DIP)
- Drug Induced Linear IgA Bullous Dermatosis (LABD)
- Stevens-Johnson Syndrome (SJS) and Toxic Epidermal Necrolysis (TEN)
- Pustular cADRs
- Acute Generalized Exanthematous Pustulosis (AGEP)
- Acute Localized Exanthematous Pustulosis (ALEP)
- Drug Induced Sub Corneal Pustular Dermatosis (SPD)
- Miscellaneous
- Drug Induced Alopecia
- Drug-Induced Photosensitivity
- Drug-Induced Vasculitis
- Severe Cutaneous Adverse Drug Reactions (ScADRs)
- Cutaneous Adverse Drug Reactions to Some Common Antibiotics
- Discussion
- Conclusion
- References
Discussion
The present review included forty-two published articles that mentioned AB or AM associated cADRs, and ScADRs. In almost all the papers, ABs were one of the major causes of cADRs or ScADRs. The lowest frequency was found to be between 5% - 10% [15, 26, 42] and highest being 81.13% [70] followed by 73.8% [71]. Several other studies also found a very high frequency of AB-associated cADRs like-Wong et al., (2019) [72] 66.1%, Dimri, Raina, Thapliyal, & Thawani, (2016) >69% [11], and Verma et al., 56% [73] (Table 2). Some of the studies did not mention the overall percentage or rate of cADRs caused by AB/AM but mentioned rates related to the individual drugs [74] (Table 2).
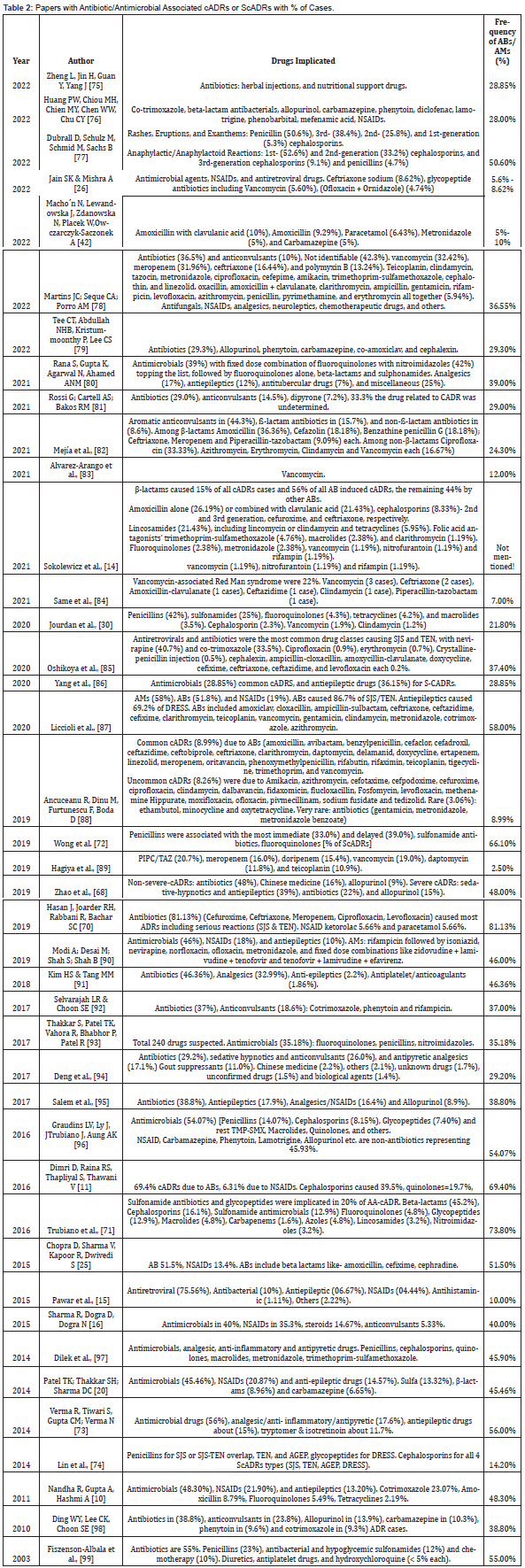
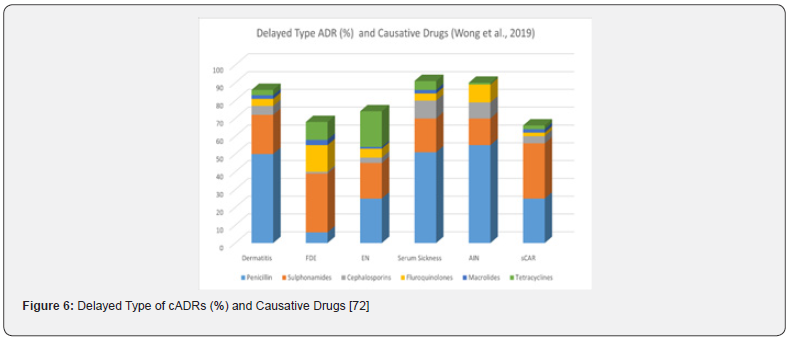
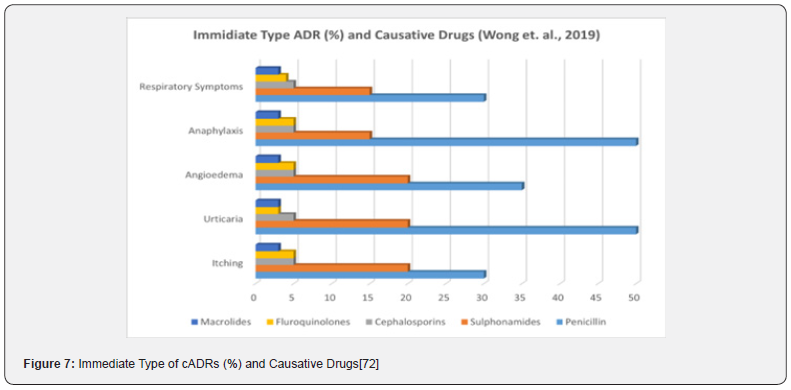
Among all the ABs, penicillins were the most implicated drug group, others being cephalosporins, sulphonamides, fluroquinolones, macrolides, glycopeptides, tetracyclines and others [42, 80, 93, 96-97, 100]. One of the articles mentioned vancomycin induced severe cADR, red man syndrome (RMS) [83]. Wong et al., (2019) [72] in their study found that penicillins were responsible for most of the immediate type cADRs (33.0%). Penicillins also caused delayed type of ScADRs (Figure 6) [72]. Penicillins were also responsible for SJS or SJS/TEN overlap in over one third of ScADRs cases [74]. Several other papers also found penicillins or other β-lactam ABs as main culprits for cADRs [77,100]. In a retrospective analysis of 608 cases in central China [68] described that ABs were responsible for both non-severe cADRs (48.0%) and severe-cADRs (22.0%) [68].
A retrospective descriptive study in a teaching hospital in Sudan found significant numbers of severe cADRs involving various antibiotics where ciprofloxacin was the greatest offender (41.46% of FDE, EM-Major, SJS and TEN). Other ABs or AMs involved were artesunate (12.2% of EM-Major, SJS, TEN), penicillin (7.32% of EM-Major, SJS), sulfamethoxazole-trimethoprim (7.32% of SJS, TEN, SJS/TEN overlap), norfloxacin (7.32% of TEN), amoxycillin (4.88% of SJS, TEN), ceftriaxone (4.88% of SJS, TEN) and ampicillin-cloxacillin, amoxicillin-clavulanic acid, Fansidar (sulfadoxine and pyrimethamine), erythromycin, clarithromycin and tetracycline caused 2.4% each of SJS, TEN and EM-Major reactions [101].
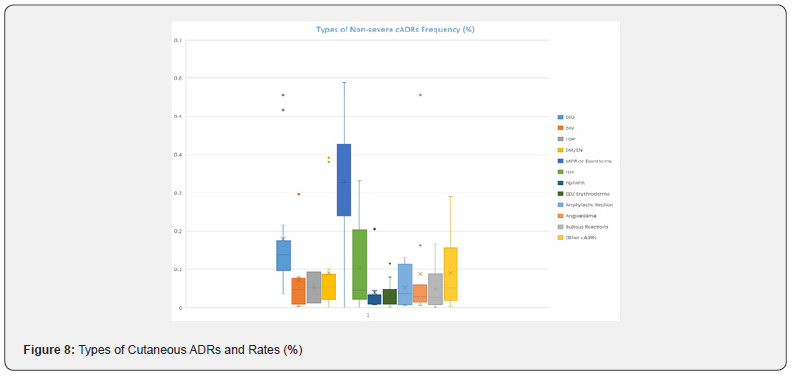
There is a wide variation in the frequency of various types of cADRs in the studies reviewed. Not all types of cADRs were observed in the range of the papers. In the present review, Maculopapular rash (MPR) or Exanthema were the most prevalent type of non-severe cADR (33.98% ± 12.72%) followed by Drug-induced urticaria (DIU) (17.36% ± 13.97%), fixed drug eruptions (FDE) (10.22% ± 10.10%), angioedema (8.79% ± 15.41%), Erythema multiforme (EM)/ Erythema nodosum (EN) (8.56% ± 11.31%), Erythema dyschromicum perstans (EDP) (5.21% ± 4.09%), Drug-induced vasculitis (DIV) (7.04% ± 8.93%), Post-drug phototoxic and photoallergic reactions (PDPAPR) 3.86% ± 5.39%), and other types (8.95% ± 8.66%) (Figure 8).
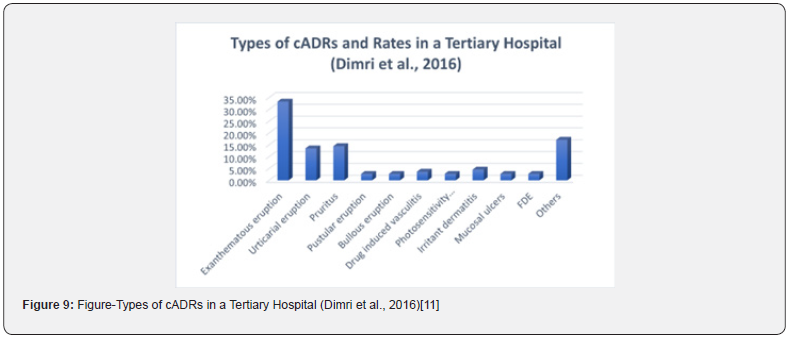
In one of the articles, rate of MPR was almost 59% [90]. Fiszenson-Albala et al., (2003) [99] also mentioned a high MPR frequency of 57%. Several others mentioned rates >40.0% [10, 77, 81, 86, 91, 102] (Table 2). Two of the papers mentioned >50.0% cADRs as DIU [91, 97]. Kim and Tang (2018) [91] also mentioned relatively higher rates of angioedema (55.67%). Dimri et al., (2016) [11] found in their retrospective analysis of cADRs reported in a tertiary care teaching hospital that the most prevalent type of skin reactions were exanthematous eruptions or MPR (33.3%) followed by Itching or Pruritus (14.4%), DIU (13.5%), other types (>17.0%) (Figure 9) [11].
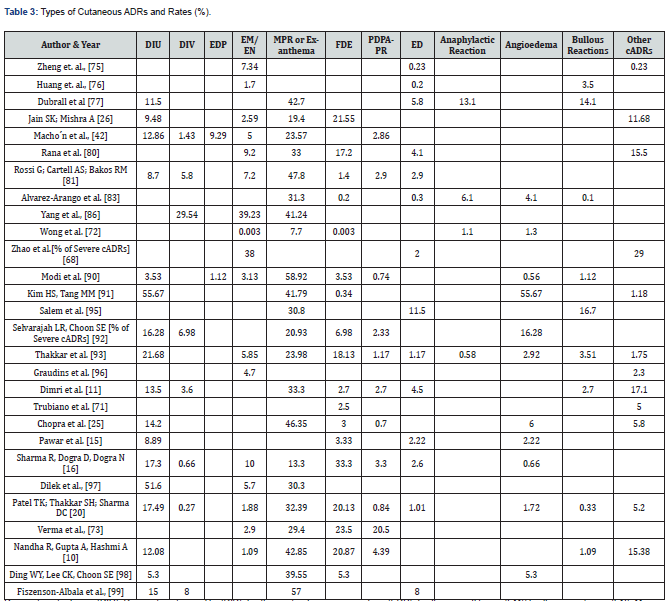
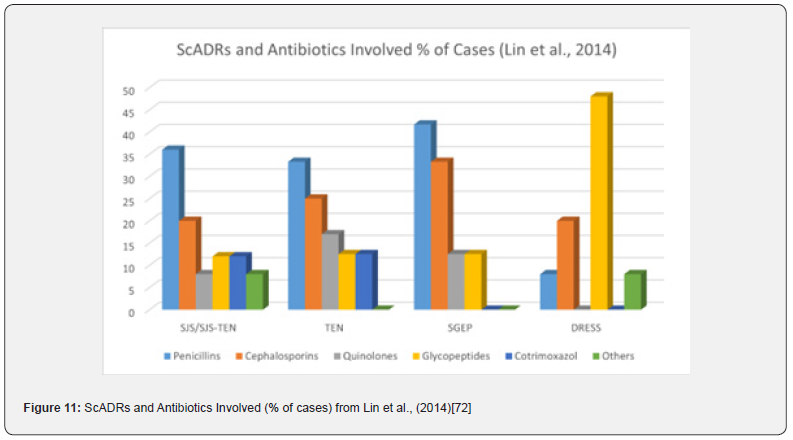
Drug-induced urticaria (DIU), Drug-induced vasculitis (DIV), Erythema dyschromicum perstans (EDP), Erythema multiforme (EM)/ Erythema nodosum (EN), Maculopapular rash (MPR) or Exanthema, Fixed drug eruption (FDE), Post-drug phototoxic and photoallergic reactions (PDPAPR), Exfoliative dermatitis (ED)/ Erythroderma, Lichenoid dermatitis (LD), Acneiform eruptions (AE), Red man syndrome (RMS) (Table 3). When it comes to severe cADRs, the most prevalent types were DRESS (18.81%), SJS/TEN overlap (14.6%), SJS (19.82%) and TEN (12.67%) (Figure 10). These were followed by AGEP, SDRIFE, and other types 7.9%, 2.86%, and 7.86% respectively (Table 4). In a Nigerian study Oshikoya et al., (2020) mentioned very high frequency of SJS (>90%) [83]. In a study at an Italian tertiary care paediatric hospital from 2010 to 2018, a very high frequency of SJS (>55%) and DRESS (>31%) was observed [87]. Rojas Mejía et al., (2021) [100] described very high rates of DRESS-DIHS (60%) in their study of ScADRs in Latin America [100]. Besides, three other studies mentioned high rates of DRESS (>30.0%) [42, 74, 92]. Among these Lin et al., (2014) [74] and Rojas Mejia et al., (2021) [100] studies were concerning ScADRs only.
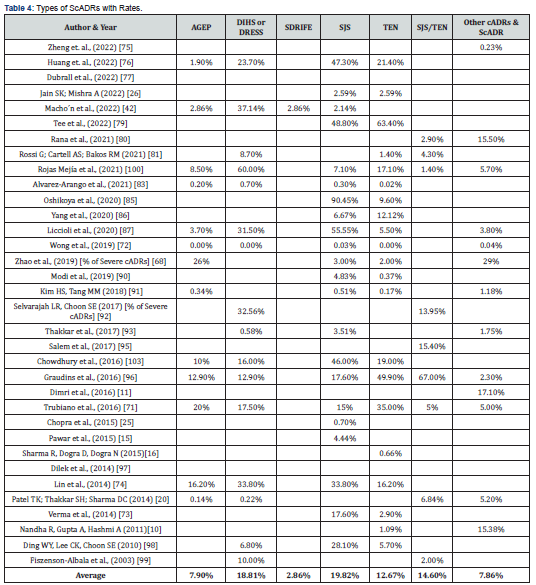
Huang et al., (2022) [76] in a retrospective analysis of 18 years ScADR data from Taiwan found very high rates of SJS, DRESS and TEN (47.3%, 23.7% and 21.4% respectively) [76]. Lin et al., (2004) [74] in their retrospective analysis of 74 cases of SCARs involving 528 patients found that 33.8% of cases had SJS, 16.2% TEN, and 16.2% AGEP [74]. Several other papers also found high rates of SJS [73, 96, 98]. Graudins, Ly, Trubiano, & Aung (2016) [96] in their ten-year follow-up of delayed cADRs found high rates of TENS (49.4%), SJS (17.6%), AGEP (12.9%), and DRESS (12.9%) and most of which were due to ABs (>50%) [96]. Lin et al., (2014) [74] described ScADRs involving penicillins, cephalosporins, quinolones, glycopeptides, cotrimoxazole and other ABs. Penicillins were responsible for highest number of cases of SJS and TEN followed by cephalosporins. Cephalosporins caused most of SGEP, and glycopeptides caused highest number of DRESS cases (Figure 11) [74]. Zhao et al., (2019) [68] too found high occurrence of AB associated ScADRs in an observational retrospective study of 608-case in central China (Figure 12) [68].

Acute generalized exanthematous pustulosis (AGEP), drug-induced hypersensitivity syndrome (DIHS) or drug reaction with eosinophilia and systemic symptoms (DRESS), symmetrical drug-related intertriginous and flexural exanthema (SDRIFE), Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), SJS-TEN overlaps, generalized bullous fixed drug eruption (GBFDE).
- Research article
- Abstract
- Background and Introduction
- Method
- Review
- Type IV hypersensitivity
- Urticarial cADRs
- Serum Sickness-Like Reaction
- Bullous Eruptions
- Drug-Induced Pemphigus (DIP)
- Drug Induced Linear IgA Bullous Dermatosis (LABD)
- Stevens-Johnson Syndrome (SJS) and Toxic Epidermal Necrolysis (TEN)
- Pustular cADRs
- Acute Generalized Exanthematous Pustulosis (AGEP)
- Acute Localized Exanthematous Pustulosis (ALEP)
- Drug Induced Sub Corneal Pustular Dermatosis (SPD)
- Miscellaneous
- Drug Induced Alopecia
- Drug-Induced Photosensitivity
- Drug-Induced Vasculitis
- Severe Cutaneous Adverse Drug Reactions (ScADRs)
- Cutaneous Adverse Drug Reactions to Some Common Antibiotics
- Discussion
- Conclusion
- References
Conclusion
Antibiotics are among the most widely prescribed and used drugs globally. This group of extensively used weapons against infection besides producing desired effects, also leads to a substantial number of undesired effects or adverse effects. Antibiotics may cause adverse effects involving almost every body system. Among all the antibiotic-associated adverse drug reactions, cutaneous drug reactions are the most prominent ones. Healthcare providers, especially physicians who treat patients, must be aware of the incidences, types, and recognition of various types of cutaneous drug reactions of antibiotics. This isn't just important for dermatologists; it's crucial for all areas of medicine that use antibiotics a lot. The present review explored the published evidence and tried to establish that antibiotics cause a significant number of cutaneous adverse drug reactions, both non-severe and severe. With the limited scope of this review, it was impossible to dig into the issue more deeply. More large-scale systematic studies could find more evidence and help the medical community understand things better.
- Research article
- Abstract
- Background and Introduction
- Method
- Review
- Type IV hypersensitivity
- Urticarial cADRs
- Serum Sickness-Like Reaction
- Bullous Eruptions
- Drug-Induced Pemphigus (DIP)
- Drug Induced Linear IgA Bullous Dermatosis (LABD)
- Stevens-Johnson Syndrome (SJS) and Toxic Epidermal Necrolysis (TEN)
- Pustular cADRs
- Acute Generalized Exanthematous Pustulosis (AGEP)
- Acute Localized Exanthematous Pustulosis (ALEP)
- Drug Induced Sub Corneal Pustular Dermatosis (SPD)
- Miscellaneous
- Drug Induced Alopecia
- Drug-Induced Photosensitivity
- Drug-Induced Vasculitis
- Severe Cutaneous Adverse Drug Reactions (ScADRs)
- Cutaneous Adverse Drug Reactions to Some Common Antibiotics
- Discussion
- Conclusion
- References
References
- World Health Organization (WHO) (2022) World Patient Safety Day 2022. Retrieved from World Health Organization, Switzerland.
- Skelly CL, Cassagnol M, Munakomi S (2022) Adverse Events Stat Pearls Treasure Island Stat Pearls Publishing.
- The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) (2016) ICH Harmonised Guideline E6(R2) Good Clinical Practice: Integrated Addendum to ICH E6(R1).
- WHO (n.d.) Medication Without Harm (2022) World Health Organization, Switzerland.
- Jo HG, Jeong K, Ryu JY, Park S, Choi YS, et al. (2022) Fatal Events Associated with Adverse Drug Reactions in the Korean National Pharmacovigilance Database J Pers Med 12(1): 5.
- Güner MD, Ekmekci PE (2019) Healthcare professionals’ pharmacovigilance knowledge and adverse drug reaction reporting behavior and factors determining the reporting rates. Journal of Drug Assessment 8(1): 13-20.
- Edwards IR, Aronson J (2000) Adverse drug reactions: definitions, diagnosis, and management. The Lancet 356(9237): 1255-1259.
- Sloane R, Osanlou O, Lewis D, Bollegala D, Maskell S, et al. (2015) social media and pharmacovigilance: A review of the opportunities and challenges Br J Clin Pharmacol 80(4): 910-920.
- Montané E, Santesmases J (2020) Adverse drug reactions. Med Clin (BARC) 154(5): 178-84.
- Nandha R, Gupta A, Hashmi A (2011) Cutaneous adverse drug reactions in a tertiary care teaching hospital: A North Indian perspective. Int J Appl Basic Med Res 1(1): 50-53.
- Dimri D, Raina RS, Thapliyal S, Thawani V (2016) Retrospective Analysis of Pattern of Cutaneous Adverse Drug Reactions in Tertiary Hospital of Pauri Garhwal. J Clin Diagn Res 10(5): FC01-FC06.
- Uthayakumar A, Oakley A (2018) Cutaneous adverse reactions to antibiotics. Gus Mitchell Maria McGivern. New Zealand: Derm Net.
- Nayak S, Acharjya B (2008) Adverse Cutanous Drug Reaction. Indian J Dermatol 53(1): 2-8.
- Sokolewicz EM, Rogowska M, Lewandowski M, Puchowska M, Piechota D, et al. (2021) Antibiotic-Related Adverse Drug Reactions in Patients Treated on the Dermatology Ward of Medical University of Gdań Antibiotics 10(10): (1144).
- Pawar MP, Pore SM, Pradhan SN, Burute SR, Bhoi UY, et al. (2015) Nevirapine: Most Common Cause of Cutaneous Adverse Drug Reactions in an Outpatient Department of a Tertiary Care Hospital. J Clin Diagn Res 9(11): FC17-FC20.
- Sharma R, Dogra D, Dogra N (2015) A study of cutaneous adverse drug reactions at a tertiary center in Jammu India. Indian Dermatol Online Journal 6(3): 168-171.
- Pozzo Magaña BR, Liy-Wong C (2022) Drugs and the skin: A concise review of cutaneous adverse drug reactions. Br J Clin Pharmacol.
- Aboud DM, Nessel TA, Hafsi W (2022) Cutaneous Adverse Drug Reaction. Stat Pearls [Internet]. Treasure Island (FL): Stat Pearls Publishing.
- Blumenthal KG, Peter JG, Trubiano JA, Phillips EJ (2019) Antibiotic allergy. Lancet 393(10167): 183-198.
- Patel TK, Thakkar SH, Sharma D (2014) Cutaneous adverse drug reactions in Indian population: A systematic review. Indian Dermatology Online Journal 5(Suppl 2): S76-S86.
- Shehab N, Lovegrove MC, Geller AI, Rose KO, Weidle NJ, et al. (2016) US Emergency Department Visits for Outpatient Adverse Drug Events 2013-2014. JAMA 316(20): 2115-2125.
- Noel MV, Sushma M, Guido S (2004) Cutaneous adverse drug reactions in hospitalized patients in a tertiary care center. Indian J Pharmacology 36(5): 292-295.
- Begum ZA, Sultana S, Umar BU, Ferdous A, Uddin MK, et al. (2012) Study of adverse drug reactions in out-patient departments of a teaching hospital. Bangladesh J Pharmacol 7(2): 104-107.
- Ata M, Hoque R, Mostafa A, Shakil MR, Biswas RS, et al. (2021) Current Status of Adverse Drug Reaction Reporting by the Physicians in A Medical College Hospital. Chattagram Maa-O-Shishu Hospital Medical College Journal 20(2): 8-13.
- Chopra D, Sharma V, Kapoor R, Dwivedi S (2015) An observational study of cutaneous adverse drug reactions in a teaching hospital. Int J Clin Pharm 37(6): 996-999.
- Jain SK, Mishra A (2022) Retrospective Analysis of Cutaneous Adverse Drug Reaction at Tertiary Care Hospital. IJPSR 13(7): 2899-2906.
- Fiszenson-Albala F, Auzerie V, Mahe E, Farinotti R, Durand-Stocco C, et al. (2003) A 6-month prospective survey of cutaneous drug reactions in a hospital setting. Br J Dermatol 149(5): 1018-1022.
- Shakib S, Caughey GE, Fok JS, Smith WB (2019) Adverse drug reaction classification by health professionals: appropriate discrimination between allergy and intolerance? Clin Transl Allergy 9(18).
- Krispinsky AJ, Shedlofsky LB, Kaffenberger BH (2020) The frequency of low risk morbilliform drug eruptions observed in patients treated with different classes of antibiotics. International Journal of Dermatology 59(6): 647-655.
- Jourdan A, Sangha B, Kim E, Nawaz S, Malik V, et al. (2020) Antibiotic hypersensitivity and adverse reactions: management and implications in clinical practice. Allergy Asthma Clin Immunol 16(6).
- Tamma PD, Avdic E, Li DX, Dzintars K, Cosgrove SE (2017) Association of Adverse Events with Antibiotic Use in Hospitalized Patients. JAMA Intern Med 177(9): 1308-1315.
- Covvey JR, Al-Balushi A, Boyter AC, Gourlay Y (2015) Antimicrobial-related medication safety incidents: a regional retrospective study in West of Scotland hospitals. J Hosp Infect 91(3): 264-270.
- Penesyan A, Gillings M, Paulsen IT (2015) Antibiotic Discovery: Combatting Bacterial Resistance in Cells and in Biofilm Communities. Molecules 20(4): 5286-5298.
- Browne AJ, Chipeta MG, Haines-Woodhouse G, Kumaran EP, Hamadani BH, et al. (2021) Global antibiotic consumption and usage in humans, 2000-18: a spatial modelling study. Lancet Planet Health 5(12): e893-e904.
- Rawlins MD (1981) Adverse reactions to drugs. Br Med J (Clin Res Ed) 282 (6268): 974-976.
- Coleman JJ, Pontefract SK (2016) Adverse drug reactions. Clinical Medicine 16(5): 481-485.
- Krska J, Cox A (2015) Adverse drug reactions.
- Thong BY (2010) Update on the Management of Antibiotic Allergy. Allergy, Allergy Asthma Immunol Res 2(2): 77- 86.
- Verman S, Anjankar A (2022) A Narrative Review of Adverse Event Detection, Monitoring, and Prevention in Indian Hospitals. Cureus 14(9): e29162.
- Marzano AV, Borghi A, Cugno M (2016) Adverse drug reactions and organ damage: The skin. Eur J Intern Med 28: 17- 24.
- Walsh S (2014) Chapter 7: Drug Rashes. In R. Morris-Jones, ABC of Dermatology. 9600 Garsington Road, Oxford, OX4 2DQ, UK: JohnWiley & Sons, Ltd 48- 54.
- Macho´n N, Lewandowska J, Zdanowska N, Placek W, Owczarczyk-Saczonek A (2022) Cutaneous Adverse Drug Reactions (CADRs)—Statistical Analysis of the Causal Relationship between the Drug, Comorbidities, Cofactors, and the Cutaneous Reaction- A Single-Centered Study. Int J Environ Res Public Health 19(13): 7982.
- Zaaroura H, Shear NH (2021) Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS). In M. Abu-Hilal, & M. Abu-Hilal (Ed.), McMaster Textbook of Internal Medicine. Cracow: Practical Medicine.
- Choudhary S, McLeod M, Torchia D, Romanelli P (2013) Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) Syndrome. J Clin Aesthet Dermatol 6(6): 31- 37.
- Akhyani M, Ghodsi ZS, Toosi S, Dabbaghian H (2005) Erythroderma: A clinical study of 97 cases. BMC Dermatol 5: 5.
- Zhu WF, Fang DR, Fang H (2021) Drug-induced erythroderma in patients with acquired immunodeficiency syndrome. World J Emerg Med. 12(4): 299- 302.
- Inamadar AC, Ragunatha S (2019) The rash that becomes an erythroderma. Clin Dermatol 37(2): 88- 98.
- Sarkar R, Garg VK (2010) Erythroderma in children. Indian J Dermatol Venereol Leprol 76(4): 341-347.
- Gulin SJ, Chiriac A (2016) Diclofenac-Induced Allergic Contact Dermatitis: A Series of Four Patients. Drug Saf Case Rep 3(1): 15.
- Gates A, Cullen S, Nykamp D (2017) Drug-Induced Hypersensitivity Reactions: Cutaneous Eruptions. US Pharmacist 42(6): 32- 36.
- Lebrec H, Bachot N, Gaspard I, Kerdine S, Guinnepain MT, et al. (1999) Mechanisms of drug-induced allergic contact dermatitis. Cell Biol Toxicol 15(1): 57- 62.
- Baigrie D, Goyal A, Crane J (2022) Leukocytoclastic Vasculitis. StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing.
- Sunderkötter C, Bonsmann G, Sindrilaru A, Luger TA (2005) Management of leukocytoclastic vasculitis. J Dermatolog Treat 16(4): 193- 206.
- Reiner MR, Brunetti VA (1997). Imipenem-cilastatin-induced leukocytoclastic vasculitis. J Am Podiatr Med Assoc 87(5): 245- 247.
- Aoud SE, Charfi N, Cheikhrouhou N, Kesentini M, Elleuch M, et al. (2015) Levofloxacin - Induced Cutaneous Leukocytoclastic Vasculitis: Report of a Case in a Diabetic Man and Review of the Litterature. Am J Epidemio Infect Dis 3(4): 84- 87.
- Mockenhaupt M (2020) Bullous Drug Reactions. Acta Derm Venereol 100(5): adv00057.
- Dyall-Smith D (2009) Bullous drug eruptions.
- Stanford CW, Kollipara R, Melookaran AM (2014) Palmoplantar Pustular Psoriasis Following Initiation of a Beta-blocker: Disease Control with Low-Dose Methotrexate. Cutis 94(3): 153-155.
- Balak DM, Hajdarbegovic E (2017) Drug-induced psoriasis: clinical perspectives. Psoriasis (Auckl) 7: 87- 94.
- Kim GK, Rosso JQ (2010) Drug-Provoked Psoriasis: Is It Drug Induced or Drug Aggravated?: understanding pathophysiology and clinical relevance. J Clin Aesthet Dermatol 3(1): 32- 38.
- Sekulovic LK, Tirnanić T (2021) Subcorneal Pustular Dermatosis. (D. M. Elston, Editor)
- Palmisano F, Annunziata MC, Mezza E, Cozzolino I, Costa C (2013) Subcorneal Pustular Dermatosis in Childhood: A Case Report and Review of the Literature. Case Reports in Dermatological Medicine.
- Solhjoo M, Goyal A, Chauhan K (2022) Drug-Induced Lupus Erythematosus Treasure Island, FL: StatPearls Publishing.
- Canadian Dermatology Association (n.d.) Alopecia. Retrieved from dermatology.
- Dyall-Smith D (2009) Alopecia from drugs.
- Piraccini BM, Iorizzo M, Rech G, Tosti A (2006) Drug-induced hair disorders. Curr Drug Saf 1(3): 301-305.
- Zhao J, Hu L, Zhang L, Zhou M, Gao L, Lin Cheng 6(2019) Causative drugs for drug-induced cutaneous reactions in central China: a 608-case analysis. An Brasile de Dermatol, 94(6): 664-670.
- Carrasco DA, Straten MV, Tyring SK (2002) A Review of Antibiotics in Dermatology. J Cut Med Surg, 6(2): 128-150.
- Hasan J, Joarder RH, Rabbani R, Bachar SC (2019) Early Event Detection and Discontinuation of the Culprit Drug Increases the Rate of Survival in Severe Cutaneous Adverse Drug Reactions: A Multi-Center Study in Bangladesh. International Journal of Sciences 8 (1): 55-59.
- Trubiano JA, Aung AK, Nguyen M, Fehily SR, Graudins L, et al. (2016) A Comparative Analysis Between Antibiotic- and Nonantibiotic-Associated Delayed Cutaneous Adverse Drug Reactions. J Allergy Clin Immunol Pract 4(6): 1187-1193.
- Wong A, Seger DL, Lai KH, Goss FR, Blumenthal KG, et al. (2019) Drug Hypersensitivity Reactions Documented in Electronic Health Records within a Large Health System. J Allergy Clin Immunol Pract, 7(4): 1253–1260.e3
- Verma R, Tiwari S, Gupta C, Verma N (2014) Cutaneous Adverse Drug Reactions-A Study of Clinical Patterns, Causality, Severity & Preventability. IOSR J Dent Med Sci 13(7): 102-109.
- Lin Y-F, Yang C-H, Sindy H, Lin J-Y, Hui C-YR, et al. (2014) Severe Cutaneous Adverse Reactions Related to Systemic Antibiotics. Clin Infect Dis, 58(10): 1377-1385.
- Zheng L, Jin H-bin, Guan Y-yao, Yang J (2022) Pharmacovigilance of cutaneous adverse drug reactions in associations with drugs and medical conditions: a retrospective study of hospitalized patients. BMC Pharmacol Toxicol. 23: 62.
- Huang P-W, Chiou M-H, Chien M-Y, Chen W-W, Chu C-Y (2022) Analysis of severe cutaneous adverse reactions (SCARs) in Taiwan drug-injury relief system: 18-year results. J Formosan Med Asso, 121(8): 1397-1405.
- Dubrall D, Schulz M, Schmid M, Sachs B (2022) Descriptive analysis of adverse drug reaction reports for hypersensitivity reactions stratified in relation to different beta-lactam antibiotics. Allergol Select 6: 42-60.
- Martins JC, Seque CA, Porro AM (2022) Clinical aspects and therapeutic approach of drug-induced adverse skin reactions in a quaternary hospital: a retrospective study with 219 cases. Anais Brasileiros de Dermatol 97(3): 284-290.
- Tee CT, Abdullah NHB, Kristummoonthy P, Lee CS (2022) Severe cutaneous adverse reactions: A 5-year retrospective study at Hospital Melaka, Malaysia, from December 2014 to February 2020. Med J Malay 77(4): 409-414.
- Rana S, Gupta K, Agarwal N, Ahamed AN (2021) A Study of Cutaneous Adverse Drug Reactions and their Association with Autoimmune Diseases at a Tertiary Centre in South-West Rajasthan, India. Indian J Dermatol, 66(4): 445.
- Rossi G, Cartell Ad, Bakos RM (2021) Dermoscopic Aspects of Cutaneous Adverse Drug Reactions. Dermatol Pract Concept, 11(1): e2021136.
- Mejía DVR, Zwiener RD, Villa RC, Ramírez LF, Espinosa DLS, et al. (2021) Severe Cutaneous Adverse Reactions to Drugs in Latin America: The RACGRAD Study. J Investig Allergol Clin Immunol 31(4): 322-331.
- Alvarez-Arango S, Yerneni S, Tang O, Zhou L, Mancini CM et al. (2021) Vancomycin Hypersensitivity Reactions Documented in Electronic Health Records. J Aller Clin Immunol Pract 9(2): 906-912.
- Same RG, Hsu AJ, Cosgrove SE, Klein EY, Amoah J, et al. (2021) Antibiotic-Associated Adverse Events in Hospitalized Children. J Ped Infec Dis Soc. 10(5): 622-628.
- Oshikoya KA, Ogunyinka IA, Ogar CK, Abiola A, Ibrahim A, et al. (2020) Severe cutaneous adverse drug reactions manifesting as Stevens-Johnson syndrome and toxic epidermal necrolysis reported to the national pharmacovigilance center in Nigeria: a database review from 2004 to 2017. Therapeut Adv Dr Saf 11: 1-18.
- Yang F, Chen Z, Chen S-A, Zhu, Q, Wang L, et al. (2020) Clinical profile of cutaneous adverse drug reactions: a retrospective study of 1883 hospitalized patients from 2007 to 2016 in Shanghai, China. Eur J Dermatol 30(1): 24-31.
- Liccioli G, Mori F, Parronchi P, Capone M, Fili L, et al. (2020) Aetiopathogenesis of severe cutaneous adverse reactions (SCARs) in children: A 9‐year experience in a tertiary care paediatric hospital setting. Clin Exp Allergy 50(1):61-73.
- Ancuceanu R, Dinu M, Furtunescu F, Boda D (2019) An inventory of medicinal products causing skin rash: Clinical and regulatory lessons. Exp Ther Med 18(6): 5061-5071.
- Hagiya H, Kokado R, Ueda A, Okuno H, Morii D, et al. (2019) Association of Adverse Drug Events with Broad-spectrum Antibiotic Use in Hospitalized Patients: A Single-center Study. Intern Med; 58(18): 2621-2625.
- Modi A, Desai M, Shah S, Shah B (2019) Analysis of Cutaneous Adverse Drug Reactions Reported at the Regional ADR Monitoring Center. Indian J Dermatol, 64(3): 250.
- Kim HS, Tang MM (2018) Cutaneous adverse drug reactions: A four-year audit from a district hospital in Johor, Malaysia. Med J Malaysia 73(6): 397-399.
- Selvarajah LR, Choon SE (2017) Incidence of cutaneous adverse drug reactions among medical inpatients of Sultanah Aminah Hospital Johor Bahru. Med J Malaysia, 72(3): 151-156.
- Thakkar S, Patel TK, Vahora R, Bhabhor P, Patel R (2017) Cutaneous Adverse Drug Reactions in a Tertiary Care Teaching Hospital in India: An Intensive Monitoring Study. Indian J Dermatol., 62 (6): 618-625.
- Deng Q, Fang X, Zeng Q, Lu J, Jing C, et al (2017) Severe cutaneous adverse drug reactions of Chinese inpatients: a meta-analysis. An Bras Dermatol. 2017; 92(3): 345-349.
- Salem B, Slim C, Fathallah R, Larif N, Zayani S, et al (2017) Clinical Pattern and Causative Agents of Adverse Cutaneous Drug Reactions (ACDRS): A 10-year Retrospective Study. Clin Therapeut, 39(8): e3-e4
- Graudins LV, Ly J, Trubiano J, Aung AK (2016) More than skin deep. Ten-year follow-up of delayed cutaneous adverse drug reactions (CADR). Br J Clin Pharmacol 82(4): 1040-1047.
- Dilek N, Özkol HU, Akbaş A, Kılınç F, Dilek AR, et al. (2014) Cutaneous drug reactions in children: a multicentric study. Postep Derm Alergol 31I (6): 368-371.
- Ding WY, Choon CK. (2010) Cutaneous adverse drug reactions seen in a tertiary hospital in Johor, Malaysia. Int J Dermatol, 49(7): 834-841.
- Finkelstein Y, Macdonald EM, Li P, Hutson JR, Juurlink DN (2014) Research Letter: Recurrence and Mortality Following Severe Cutaneous Adverse Reactions. JAMA 311(21): 2231-2232.
- Rojas Mejía DV, Zwiener RD, Villa RC, Ramírez LF, Espinosa DL, et al. (2021) Severe Cutaneous Adverse Reactions to Drugs in Latin America: The RACGRAD Study. J Investig Allergol Clin Immunol 31(4): 322-331.
- Shalayel MH, Ayed IA, Huneif MA, Kordofani YM (2018) A Retrospective Evaluation of Cutaneous Adverse Drug Reactions (CADRs) Due to Antibiotics Using Naranjo Adverse Drug Reactions (ADRs) Probability Scale. J Young Pharm 10(1): 113-116.
- Kim HS, Tang MM (2018) Cutaneous adverse drug reactions: A four-year audit from a district hospital in Johor, Malaysia. Med J Malaysia, 73(6): 397-399.
- Chowdhury MNG, Hoque ME, Khan MAL, Khan MSI (2016) Severe Cutaneous Adverse Drug Reactions in Bangladesh: A Review in a Tertiary Level Hospital. J Arm Forc Med Coll, Bang. 12(2): 71-75. https://doi.org/10.3329/jafmc.v12i2.41098






























