Rare Presentation of Metastatic Hepatocellular Carcinoma to The Finger, Case report and Review of the literature
Rhana Mousavi F1, Bassem Bekheit2, Nick A Hirasd3, Emon Alavi4 and Abanoub Gabra1*
1Department of Pathology and Laboratory Medicine, HCA East Florida, USA
2Dr Kiran C Patel School of Medicine, NOVA Southeastern University, USA
3Florida Atlantic University, Florida, USA
4Philadelphia College of Osteopathic Medicine South Georgia, USA
Submission: February 14, 2023;Published: March 30, 2023
*Corresponding author: Abanoub Gabra, Department of Pathology and Laboratory Medicine, HCA East Florida, USA
How to cite this article: Rhana Mousavi F, Bassem Bekheit, Nick A Hirasd, Emon Alavi and Abanoub Gabra. Rare Presentation of Metastatic Hepatocellular Carcinoma to The Finger, Case report and Review of the literature. JOJ Dermatol & Cosmet. 2023; 5(3): 555662. DOI: 10.19080/JOJDC.2023.05.555662
Abstract
Hepatocellular carcinoma (HCC) is a primary tumor of the liver that develops in the setting of chronic liver disease. HCC is difficult to diagnosis due to it requiring the use of multiple imaging modalities with the goal to detect tumors when they are less than or equal to 2 cm in size to allow all possible treatment options to be used. Herein, we discuss a 69-year-old male with stage IV liver cancer residing in hospice presenting with left middle finger osteomyelitis and extreme pain. Radiology revealed a destructive lesion, but pathology provided the diagnosis of metastatic hepatocellular carcinoma in his finger. Even though HCC can be diagnosed on imaging alone, this case highlights the need for biopsy to provide an accurate diagnosis.
Keywords: Hepatocellular Carcinoma; Prominent nucleoli; Melanoma; Liver cirrhosis; Metastasis
Introduction
Hepatocellular carcinoma (HCC) is the most common (>80%) primary liver malignancy worldwide and 80% of HCC cases arise in cirrhosis [1]. Hepatocellular carcinoma is the sixth most common malignancy and the fourth most common cause of cancer mortality worldwide [2]. The median age of onset is in the sixth decade with a male to female ratio of 3 to 1 [3]. Risk factors includes chronic liver disease leading to cirrhosis primarily due to Chronic Viral Hepatitis (HBV and HCV), heavy alcohol consumption, nonalcoholic fatty liver disease, environmental exposures such as aflatoxins, and developmental/congenital disorders such as ataxia telangiectasia and Alagille syndrome [4]. Prognosis for a five-year overall survival is 24-70% and depends mainly on TMN staging [5]. A poor prognosis is associated with lympho-vascular invasion, presence of cirrhosis, multifocality, tumor size > 2 cm, and portal vein thrombosis [3].
Case Presentation
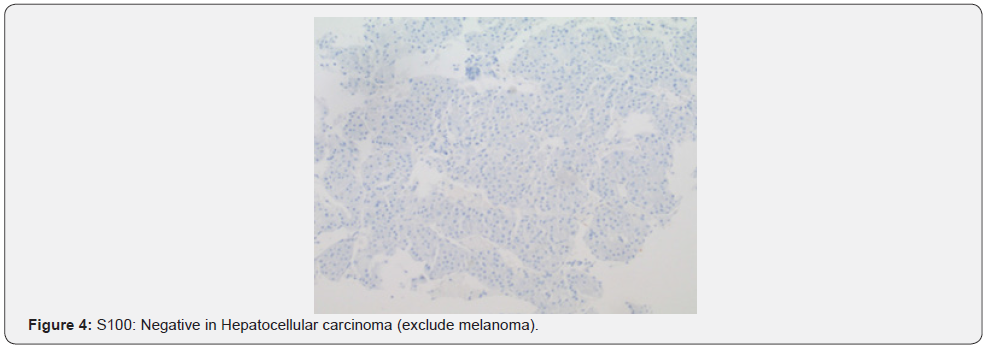
A 69-year-old male presented to the emergency department with a chief complaint of left middle finger osteomyelitis and extreme pain. He was in hospice for a stage IV liver cancer which arose in his cirrhotic liver five years ago. The patient’s finger was swollen and had undergone a course of unknown antibiotics orally for four weeks without any improvement. The patient was afebrile with jaundice at the time of admission with normal white blood cell count and elevated liver function tests. He had total bilirubin of 3.6 (mg/dl), AST of 186 (U/L), ALT 174 (U/L), and alkaline phosphatase of 230 (U/L). He denied alcohol and recreational drugs use. Radiology report of his left hand revealed a destructive lesion of left third finger with differential diagnosis of osteomyelitis. On physical exam, his finger was tender to palpitation with no other significant findings. A biopsy of the lesion sent for pathology department concluded the final diagnosis of as metastatic hepatocellular carcinoma. Tumor cells resemble hepatocytes with polygonal shape, round, vesicular nuclei, and prominent nucleoli. Inclusions are seen in tumor cells: Mallory hyaline, ground-glass inclusions, and hyaline globules. Clear cells present and even numerous due to accumulation of glycogen or fat. Bizarre mono- or multinucleate tumor giant cells, rare osteoclast-like giant cells also present. Tumor cells are positive for Hepar and negative for cytokeratin and S100 which confirm the diagnosis of hepatocellular carcinoma. He had Surgical intervention by partial left lobectomy 10 years ago following systemic chemotherapy with Sorafenib a tyrosine kinase where relapsed as a finger nodule (Figure 1).
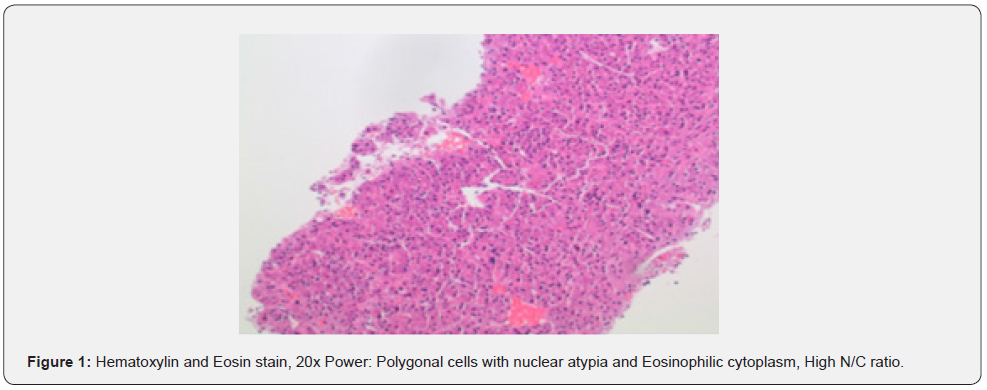
Discussion
Hepatocellular carcinoma (HCC) is the most common cause of primary hepatic malignancy, and it is the second most common cause of cancer related death [2]. In the US, hepatitis C is the largest risk factor for developing HCC while in regions such as Africa and Asia, hepatitis B is the biggest risk factor [2]. Aside from hepatitis and cirrhosis, there are other less common conditions that can increase the risk of developing HCC by causing chronic injury and fibrosis to the liver, these conditions include aflatoxin from aspergillus, alpha 1 antitrypsin deficiency, Wilson’s disease, and (glycogen & lysosome) storage diseases [2]. There are some studies suggesting that metabolic syndrome may contribute to HCC but on the other hand, research from Japan has shown that nonalcoholic fatty liver disease is not a major risk factor especially if there was no cirrhosis involved [6].
Metastasis of HCC was long believed to be rare event due to lower 5-year survival in those patients [3]. In era of advanced treatment modalities, HCC has a higher propensity for metastasis with many patients having more metastatic lesions as found on autopsies [3]. Autopsies of those with HCC showed that as many as 78% of patients have lesions outside the liver including in the lungs and lymph nodes [7].
Those at increased risk for intrahepatic recurrence should be screened with Ultrasound every 6 months [2,8] and if suspicious lesion is identified in ultrasound, then follow up imaging with CT and MRI may be required [2]. In certain cases, biopsy may be necessary to make the diagnosis [2]. Screening using alpha fetoprotein is not recommended because it lacks specificity, sensitivity and remains controversial [2]. AFP is elevated in many other conditions such as cholangiocarcinoma, embryogenic tumors, and chronic hepatitis [4]. Its use while debated has been incorporated in the different staging classifications of HCC including Barcelona Clinic Liver Cancer Staging (BCLC) and Hong Kong Liver Cancer Staging (HKLC). When evaluating a liver Ultrasound on high-risk patients with known history of hepatocellular carcinoma, a 1 cm liver lesion is considered suspicious and should be worked up with a CT scan [2]. An MRI may be considered as next line imaging because of its high sensitivity and lack of radiation [2]. It is important to consider the increased cost and higher chance of artifact that comes with MRIs [6].
Prevention of HCC comes down to preventing the underlying liver injury that led to the cirrhosis and hence the HCC [2]. Given that HCV is the largest risk factor in the US for developing HCC, it follows that successful treatment and eradication of HCV would greatly reduce the incidence of HCC [2]. Fortunately, Hepatitis C treatment has seen major developments in recent years, Interferon and ribavirin were previously the only known medications for HCV but now there is a new class of drugs called direct acting antivirals (DAAs) that have 100% response rate according to some studies [2,6]. Despite advances in HCV treatment, the true impact is predicated to be a meek decrease of 10-15% of HCC. This is because many patients with HCV remain undiagnosed and the treatment is not accessible to those of lower socioeconomic status [1]. Meanwhile, worldwide vaccination against HBV has demonstrated to reduce the incidence of HCC as well as the use of lamivudine [2]. Early interventions in other conditions such as alcohol cessation, or new treatment for aspergillus, Wilsons, and storage disease would also curtail the disease burden [6] (Figure 1).
Treatment for HCC depends on the spread and pattern of extent. There are four major modalities by which HCC can be managed [2]: surgical resection, regional therapy, transplant, and systemic therapy. If a patient has a unifocal nonmetastatic noncirrhotic HCC then the patient would be a good candidate for surgical resection due to the lack of cirrhosis in the spared tissue that could become malignant [2]. Consider regional modalities when HCC is multifocal. Regional therapy can be used for patients with HCC less than 3 cm and would include radiofrequency, transcatheter arterial chemoembolization (TRACE), and Ytterium-90 [2]. Liver transplant is another option for patients who are abstinent from alcohol and meet the Milan criteria. The Milan criteria requires a single HCC lesion less than 5 cm or 2-3 HCC lesions less than 3 cm [2]. If the patient has metastasis outside the liver as in our case report, then systemic therapy would be indicated which includes the newly FDA-approved sorafenib which functions as a VEGF multi-kinase inhibitor that slows the progression of HCC [6].
Acral metastasis is defined as metastasis to extremities and mostly presents in those with a known history of malignancy [5]. Only 10% of presentations have acrometstasis as the first symptom [5]. First reported case was hand acrometastasis in 1906 by Handley [9]. Other early reported cases include HCC to the thumb in 1970 where the digit was amputated but the patient died 7 months later. Another reported case in 1988 of HCC metastasis to the first metacarpophalangeal joint and was resected but patient passed away after 15 months [3]. A literature review for cases of acro metastasis through Cochrane, Medline, and PubMed found 70 case reports of which 49 had hand metastasis and 21 had foot metastasis, men are affected at twice the rate of women and the most common cause is lung malignancy [5]. Breast cancer was found to spread equally to hands and feet [5]. Renal cell carcinoma was more common in foot metastasis [5]. The dominant hand was mostly involved, and the 3rd digit was the most affected [5]. Surgical resection (amputation) was the most common treatment, but reoccurrence was as high as 20% [9] (Figure 2).
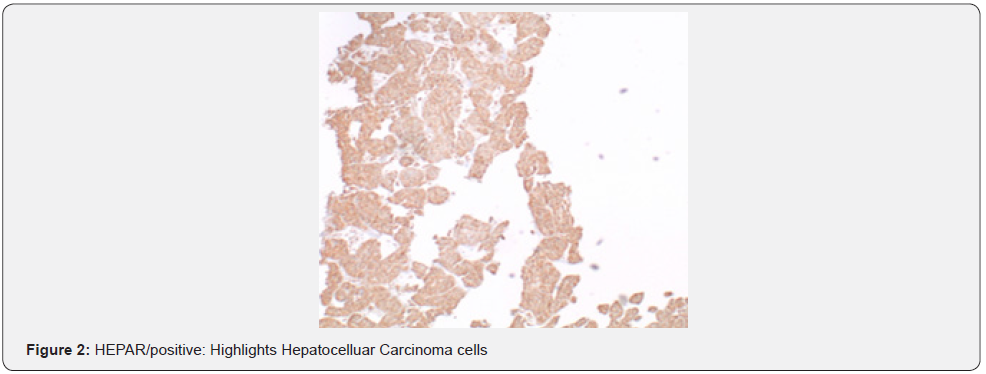
There have been numerous case reports about metastasis to the extremities from HCC and other malignancies. Cutaneous manifestation of HCC is rare but there was a reported case of a 37-year-old with HCC metastasis to his scalp and middle finger after he received TRACE. Patient survival was not reported as he was being monitored at the time [10]. Another case involved a patient with known history of liver cirrhosis who was diagnosed with HCC presented with lesion in his left thumb that was treated initially as an abscess with I&D and antibiotics. He didn’t respond to initial therapy and further workup showed HCC metastasis. Patient died 5 months later from complications of metastasis. Histology of his lesion showed bile canaliculi, bile secretion, and cells in sinusoidal arrangement [7].
Another case involved a 55-year-old male with HBV and HCC with known metastasis to spleen and lung came into dermatology for a new hand lesion. Dermatology was concerned for pyogenic granuloma, but biopsy showed HCC metastasis. Amputation of the digit followed but the patient died 6 months later [3].

Taking detailed history is crucial to rule in/out other metastasis. For example, A case was reported in the literature of 53-year-old female with known history of oropharyngeal squamous cell carcinoma in the left index finger which was treated as acute gouty arthritis with prednisone in the outpatient setting [11]. Another case of a 72 y/o male with smoking history diagnosed with bronchogenic carcinoma was found to have metastasis to the right big toe [12] (Figure 3).
HCC has an average survival length of 36 months [2]. Moreover, Metastasis to skin and soft tissue is a poor prognostic indicator and studies by Damron and Heiner’s reports survival between 4-18 month while other studies suggest 6 months [9,13].
Metastatic tumors to the digits are commonly caused by lung, GU, and breast malignancy [13]. Of those 0.1%, 33% was lung cancer, 20% RCC, 13% breast cancer, and 10% colon cancer [9]. One case of a 44-year-old male with esophageal carcinoma metastasis to the digit died 2 months later [13]. Another patient has lung cancer and was found to have thumb metastasis. Patient underwent radiation therapy and deceased 6 months later [14].
Acral metastasis is a rare occurrence and subungual metastasis represents 1% of acral metastasis. There has been a reported case of a 72-year-old patient with lung cancer who had subungual lesion on his 3rd finger suspicious of infection that was found to be metastasis per biopsy [12]. There was another reported case of subungual lesion on the left big toe of a 57-year-old that was found to be metastasis of urothelial carcinoma [12]. Both presentations had erythematous swelling of the distal finger that distorted the nail plate [15]. After reviewing case reports, it is likely that skin metastasis happens in late-stage advanced malignancies and indicates worse prognosis. These cases underscore the importance of clinicians being highly suspicious of new skin lesions in the extremities in patients with a given history of malignancy and that metastasis should be high on the differential [7,15] (Figure 4).
Morphologically, other hepatic and non-hepatic lesions can mimic hepatocellular carcinoma metastasis. Cholangiocarcinoma has discrete glands and desmoplastic stroma, Mucicarmine-CK7- CK19- MOC31 are often positive, Hepatocyte nuclear factor-β highlights most cholangiocarcinoma’s while most HCCs are negative, and Hepatocellular immunohistochemical markers are negative [16]. Typical mutational profile can help in making diagnosis in challenging cases as TERT promoter and CTNNB1 mutation favor HCC and IDH1/IDH2 mutation, FGFR2 fusions favor intrahepatic cholangiocarcinoma [17]. Metastatic Neuroendocrine Tumor has prominent collagenous stroma and is positive staining for neuroendocrine markers, Hepatocellular markers negative; rare cases can show aberrant staining with HepPar1 [18]. In Metastatic Adenocarcinoma, Mucicarmine, MOC31 are positive; keratin profile not limited to 8 and 18 [19]. Metastatic Renal Cell Carcinoma should be suspected in patients with history of RCC or renal tumor, keeping in mind that RCC is well known for metastasizing to unusual areas of body include Thyroid gland [20].
Arginase-1, HepPar1 are negative while PAX2 and PAX8 are positive [20]. In case of Combined Hepatocellular- Cholangiocarcinoma, Additional cholangiocarcinoma component defined by discrete gland formation and strong staining for CK7, CK19, &/or MOC31 in presence of morphological evidence of both carcinomas [21].
Metastatic Adrenocortical Carcinoma in which PAN-Keratin is weak or absent, hepatocellular markers negative but the tumor cells are positive for SF1, inhibin, calretinin, &/or MART-1 [22].
Hepatoid Carcinoma is aggressive tumor with poor outcome which represents rare extrahepatic tumor resembling HCC on morphology and immunohistochemistry with no definite hepatic lesions except in case hepatoid carcinoma metastasizing to liver (1). Hepatocellular markers, including arginase-1, AFP, and glypican-3, can be positive besides CK19 and CK20 can be also positive.
Conclusion
Hepatocellular carcinoma (HCC) is a primary tumor of the liver that primarily develops in the setting of chronic liver disease. HCC is difficult to diagnosis due to it requiring the use of multiple imaging modalities with the goal to detect tumors when they are less than or equal to 2 cm in size to allow all possible treatment options to be used. We presented a 69-year-old male with stage IV liver cancer residing in hospice presenting with left middle finger osteomyelitis and extreme pain which was biopsied and confirmed as metastasized hepatocellular carcinoma. Awareness of the potentiality of hepatocellular carcinoma to present and mimic osteomyelitis would help clinicians and pathologists to have HCC in differential diagnosis.
References
- Miyama Y, Fujii T, Murase K, Takaya H, Kondo F (2020) Hepatoid adenocarcinoma of the lung mimicking metastatic hepatocellular carcinoma. Autops Case Rep 10(2): e2020162.
- Liovet JM, Zucman-Rossi J, Pikarsky E, Sangro B, Schwartz M, Sherman M, et al (2012) Hepatocellular carcinoma.
- Kim J Il, Song CH, Gong HS (2012) Finger skin metastasis from hepatocellular carcinoma: a case report. Hand Surg 17(1): 131-134.
- Wang H, Chen L (2013) Tumor microenvironment and hepatocellular carcinoma metastasis. Vol. 28, Journal of Gastroenterology and Hepatology (Australia). Blackwell Publishing 43-48.
- Hartke J, Johnson M, Ghabril M. The diagnosis and treatment of hepatocellular carcinoma. Semin Diagn Pathol 34(2):153-159.
- Clark T, Maximin S, Meier J, Pokharel S, Bhargava P (2015) Hepatocellular Carcinoma: Review of Epidemiology, Screening, Imaging Diagnosis, Response Assessment, and Treatment Curr Probl Diagn Radiol 44(6): 479-486.
- Lee KS, Lee SH, Kang KH, Oh KJ (1999) Original Articles Metastatic Hepatocellular Carcinoma of The Distal Phalanx of the Thumb: A Case Report. Hand Surg 4(1): 95-100.
- Piñero F, Dirchwolf M, Pessôa MG (2020) Biomarkers in Hepatocellular Carcinoma: Diagnosis, Prognosis and Treatment Response Assessment. NLM (Medline) 9(6): 1370.
- Stomeo D, Tulli A, Ziranu A, Perisano C, de Santis V, et al. (2015) Acrometastasis: a literature review. Eur Rev Med Pharmacol Sci 19(15): 2906-2915.
- Yan J, Deng M, Fan H (2022) Scalp and Finger Metastasis from Hepatocellular Carcinoma. Clinical Gastroenterology and Hepatology 20(9): 31-32.
- Sandberg Y, van de Wiel BA, van Meerten E (2015) Digital acrometastasis. Arthritis Rheumatol 67(5): 1410.
- Khashaba A (2000) Acrometastasis. American Journal of Emergency Medicine 18(4): 502–502.
- Chen Y, Tang W, Xiao H, Chen J, Zhao H, et al. (2017) An isolated unusual digit metastasis from esophageal carcinoma: A case report. Onco Targets Ther 10: 2449-2452.
- Nakamura H, Shimizu T, Kodama K, Shimizu H (2005) Metastasis of lung cancer to the finger: A report of two cases. Int J Dermatol 44(1): 47-49.
- Pistone G, Tilotta G, Gurreri R, Castelli E, Curiale S, et al. (2019) Possible role of polycyclic aromatic hydrocarbons in the onset of melanoma: preliminary data. J Eur Acad Dermatol Venereol 33(8): e280-e282.
- Razumilava N, Gores GJ (2014) Cholangiocarcinoma. The Lancet. 383 (9935): 2168-2179.
- Chaisaingmongkol J, Budhu A, Dang H, Rabibhadana S, Pupacdi B, Kwon SM, et al. (2017) Common Molecular Subtypes Among Asian Hepatocellular Carcinoma and Cholangiocarcinoma. Cancer Cell 32(1): 57-70.
- Gangi A, Howe JR (2020) The Landmark Series: Neuroendocrine Tumor Liver Metastases. Ann Surg Oncol 27(9): 3270-3280.
- Hammar SP (1998) Metastatic adenocarcinoma of unknown primary origin. Hum Pathol 29(12): 1393-1402.
- Overby A, Duval L, Ladekarl M, Laursen BE, Donskov F (2019) Carcinoma of Unknown Primary Site (CUP) With Metastatic Renal-Cell Carcinoma (mRCC) Histologic and Immunohistochemical Characteristics (CUP-mRCC): Results from Consecutive Patients Treated with Targeted Therapy and Review of Literature. Clin Genitourin Cancer 17(1): e32-37.
- Connell LC, Harding JJ, Shia J, Abou-Alfa GK (2016) Combined intrahepatic cholangiocarcinoma and hepatocellular carcinoma. Vol. 5, Chinese Clinical Oncology. AME Publishing Company.
- van Ditzhuijsen CIM, van de Weijer R, Haak HR. Adrenocortical carcinoma. Neth J Med 65(2): 55-60.






























