Research on Nitrosamines in Cosmetics: A Review
Yingjie Zhu1, Yuan Xia1, Zhanghao Chen2 and Liangliang Lin1*
1Key Laboratory of Synthetic and Biological Colloids, Ministry of Education, School of Chemical and Material Engineering, Jiangnan University, China
2Guangdong Institute for Drug Control, NMPA Key Laboratory for Safety Risk Assessment of Cosmetics, China
Submission: December 19, 2022;Published: January 05, 2023
*Corresponding author: Liangliang Lin, Key Laboratory of Synthetic and Biological Colloids, Ministry of Education, School of Chemical and Material Engineering, Jiangnan University, China
How to cite this article: Yingjie Z, Yuan X, Zhanghao C, Liangliang L. Research on Nitrosamines in Cosmetics: A Review. JOJ Dermatol & Cosmet. 2023; 5(2): 555656. DOI: 10.19080/JOJDC.2023.05.555656
Abstract
As a kind of toxic and strongly carcinogenic substance, nitrosamines are likely to be present in the raw materials of cosmetics, or during the preparation, storage, and transportation processes. This would result in the potential hazard to the health of consumers. This paper carries out the study on nitrosamines. Firstly, the physical and chemical properties of nitrosamines as well as their types in cosmetics are introduced. Then, the formation reasons and possible reaction mechanisms of nitrosamines in cosmetics are summarized. Next, various detection methods of nitrosamines are concluded and compared. The legislation, norms, and standards for nitrosamines in cosmetics around the world are listed. Finally, the development trends of detection, supervision, and management for nitrosoamines in cosmetics are predicted.
Keywords: Nitrosamines; Cosmetics; Toxic substances; Nitrosamine detection; Cosmetic hazards
Introduction
Economic development and the pursuit of beauty make the cosmetics market vigorous, and cosmetics are essential for the peoples in their daily life. Due to the strong purchasing power, China has become the second largest market of cosmetics in the world. It has been reported by National Bureau of Statistics that the total retail sales of cosmetics of China in 2021 reached 402.6 billion yuan, increasing by 18.41% in comparison with 340 billion yuan in 2020. Hence, the average annual rate is higher than that of other countries. Moreover, the number of licensed cosmetics manufacturers is 5,400, and the number of products with legal licenses reaches up to 1.6 million. Hence, the cosmetics have a wider develop space in China [1]. As is well known, cosmetics are a kind of mixture produced according to scientific formulations. During the processes of production, processing, storage and transportation, some harmful substances could be generated or brought into, thus posing a threat to the consumers’ health. Among them, nitrosamines are the common hazards with obvious carcinogenicity, as well as easily produced by the reaction between precursor secondary amine and nitrite. Simultaneously, the reaction can progress well under the natural conditions or in the human and animal bodies. They can enter our bodies via the respiratory tract, digestive tract, or skin. Moreover, not only can the long-term low dose cause cancer, but also a shock of higher dose can directly result in carcinogenesis [2]. Therefore, the nitrosamines harm has undoubtedly been paid close attention to. Moreover, it is of great research significance and application value to know the sources, influence factors, formation mechanisms and detection methods of nitrosamines in cosmetics to effectively inhibit the formation of nitrosamines.
Nitrosamines Profile
The nitrosamines have significant carcinogenicity, which is related to their chemical structures, physical and chemical properties, tissues and organs, metabolic process, etc. However, until now, the specific mechanism has still not been clear. The existing findings suggest that the increase of electron-withdrawing ability of hydrogen at α-C (R1 and R2) attached to ammonia nitrogen decreases the electron cloud and density of α-C and N-nitroso-group and increases the number of static charges. This would make the hydrogens on the carbon atom more reactive, and easily oxidized, decomposed, isomerized to alkyldiazohydroxides in vivo. As a highly active carcinogen, this compound can block DNA replication and induce gene mutation [3]. Furthermore, some researchers have found that the carcinogenicity of nitrosamines might be related to the free radical activity. For example, Hirata et al. have shown that the N-nitrosamine compounds in the tobacco could induce the generation of reactive oxygen species, stimulate the Wnt signal path, and thus resulting in the lung cancer.
All the countries issue the standards for the contents of nitrosamines in cosmetics. However, there are still many cosmetics with a lower content of nitrosamines, and then they can’t be analyzed and detected at the present stage. Furthermore, due to the complex raw materials and limited detection means, the generation condition and formation mechanism are still unknown, which further increases the difficulty to effectively inhibit the content of nitrosamines in cosmetics. In this paper, the types of nitrosamines, generation causes, reaction mechanisms, detection means, regulations and standards in various countries are summarized, so as to provide the references for relevant manufacturers, consumers, supervisors, and researchers.
Types and Sources of Nitrosamines
Types of nitrosamines
There are many types of nitrosamines in cosmetics (Figure 1), of which the most common one is N-Nitrosodiethanolamine (NDELA). In 1997, the Food and Drug Administration (FDA) first reported that the detection rate of NDELA in cosmetics was up to 31% with the content of 1~130000 μg/kg [4,5]. In 1986, 2-isoctyl-4-(N-nitroso-N-methylamino) benzoic acid (NMPABAO) was detected in the sunscreens, as well as a familiar type of nitrosamine. Notably, with more attention to the cancer risk of nitrosamines and the progress of detection technology, more than 300 types of nitrosamines have been detected in cosmetics. The frequently detected ones are listed in Table 1.
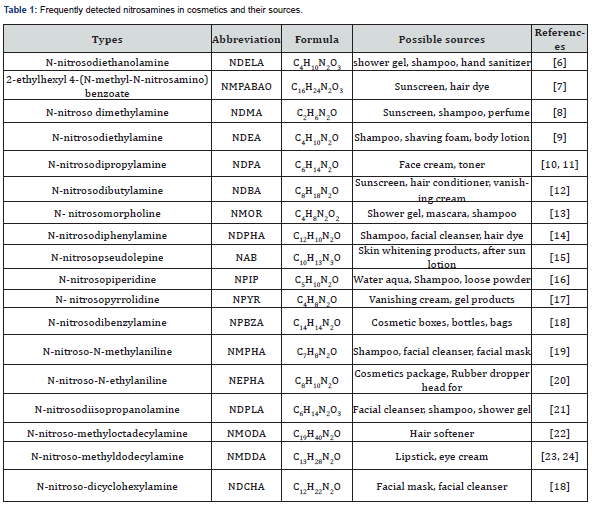
Sources of Nitrosamines
Raw materials: Since nitrosamines were detected in cosmetics in 1977 for the first time, most researchers regard the raw materials as an import source of the nitrosamines. From 1980 to 1984, FDA detected 1 mg/kg of nitrosamines in about 30% cosmetics [25]. Joo et al. [26] detected NDELA in multiple raw materials of cosmetics (triethanolamine, trimethylamine, coconut fatty acid diethanolamide, fatty acid chain alkanolamide, and triethanolamine-dodecyl sulfate) with high-performance Liquid Chromatography-tandem Mass Spectrometry (HPLCMS- MS) method. Guo Huifang [27] analyzed the several typical nitrogen-containing surfactants in cosmetics, such as coconolic acid monoethanolamide (CMEA), cocoanut fatty acid n, n-diethanolamide (CDEA), and castor oil acylthreonine sodium, simultaneously, established an efficient and simple method to detect the NDELA in nitrogen-containing surfactants.
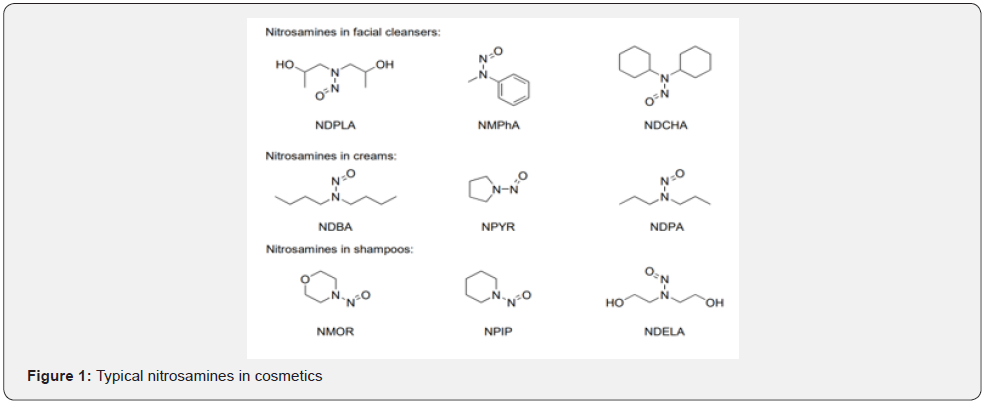
In 2014, the detection limit and quantification limit of volatile nitrosamines in ten types of cosmetics were determined in China [28]. However, the complex raw materials of cosmetics could result in the increase of the types of hazards introducing the nitrosamines. Cocoamidopropyl betaine (CAB) is common in cosmetics, and widely used in washing products such as shower gel, shampoo, and hand sanitizer. It has a quaternary ammonium group with strong electronic induction, which makes the carboxylic acid group attached to the quaternary nitrogen atom always present in a salt-like group, and thus showing amphoteric ions characteristics. Hence, CAB is a kind of better surfactant [29]. The synthesis of CAB includes three steps, that is, synthesis of cocamidopropyl dimethylamine, neutralization between chloroacetic acid aqueous solution and alkali, and synthesis of coir amide propyl betaine, as shown in Figure 2.
Both the raw materials for synthetizing CAB and intermediate products have the tertiary amine structure. Moreover, once the tertiary amine structure contacts with the nitrosation reagents such as nitrite and nitrate, the nitrification reaction would occur. Therefore, there would possibly form a tiny amount of nitrosamines during the synthesis process of CAB, resulting in the potential risk of nitrosamines in the products.
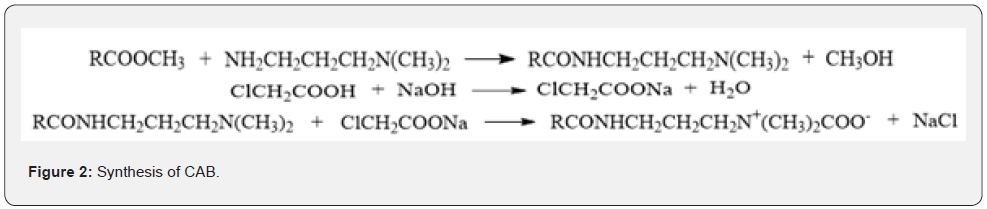
Preparation process: In addition to the raw materials, the preparation process of cosmetics is also an important source of nitrosamines. Under the effect of nitrosification reagents, the precursors (primary amine, secondary amine, and tertiary amine) can form nitrosamine compounds. Among them, the secondary amine is more prone to nitrosation reaction [30]. At present, there are many nitrosation reagents, i.e., HNO2, NO, NO2, N2O3, N2O4, NOX (X=Cl and Br), various nitrates, and nitrites. If the cosmetics contain diethanol amine DEA), triethanolamine (TEA), nitrosation agent, and fatty acid alkanolamide as the emulsifier or pH modifier, there would form nitrosoamines under certain conditions [31]. As such, the formation mechanism relates with pH. Under acidic conditions, HNO2 can directly react with amines, or two HNO2 molecules can react with each other to form N2O3 and H2O. Then, N2O3 forms nitrosamine after one-step reaction. For example, the nitrosation reaction of N, N-dimethylaniline can progress under the acidic condition, and this reaction can be divided into two steps (Figure 3). Firstly, the nitrous acid forms the nitrosyl cation in an acidic environment. Subsequently, the nitrosyl cation attacks the nitrogen atoms as the electrophile, thus forming the nitroso cation intermediate of nitrogen. Then, according to the different classifications of amine, the substituents attached to nitrogen atom with positive charge of intermediate are different. When the hydrogen atoms are attached to nitrogen atoms of primary amine and secondary amine, they can be directly removed to form nitrosamine. On the contrary, there are no hydrogen atoms attached to the nitrogen atoms, so failing in dehydrogenation. However, the dimethylamino is an active group, and the nitroso cation can form C-Nitroso compound through the electrophilic substitution on the para-position of the benzene ring [31].
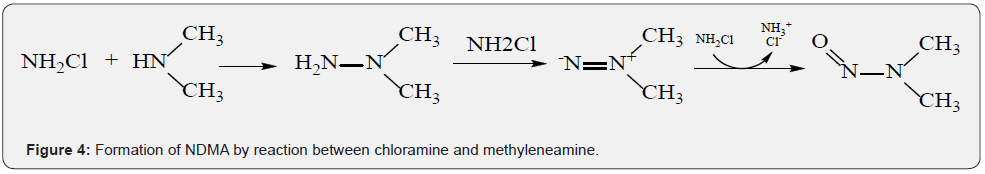
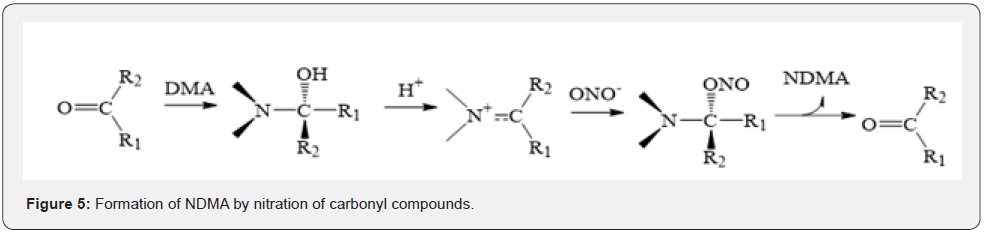
Nitrosamines are relatively stable under non-acidic conditions, whereas the resonant structure resulting from conjugated effect between lone pair electrons and nitroso could make the H attached to α-C easily oxidized. Moreover, as the electron withdrawing group is attached toα-C, the electron cloud densities of α-C and N-NO would be decreased. Simultaneously, the H attached to α-C would be more active and easily be oxidized. In 2002, CHOI et al. [32] have reported that NDMA could be formed in the waste water with chloramine, and simultaneously, they proposed the reaction mechanism (Figure 4). Notably, DMA can react with NO2- to form NDMA under the catalysis effect of formaldehyde, trichloroacetaldehyde, and carbonyl compound such as acetaldehyde, acetone and trifluoroacetaldehyde [33]. Bromonitropropanol is the common preservative and fungicide in cosmetics with the content of 0.04%~0.1%. When pH is 8~12, Br-, NO2- and formaldehyde could be released from bromonitropropanol after decomposition, and then catalyzes their reactions with DMA to form NDMA (Figure 5). Hence, our law requires that the content of bromonitropropanol should not exceed 0.1%.
Storage process: Generally, the preservatives and stabilizers are added to cosmetics for the long-term storage and good performance. The surfactant, viscosifier, pH regulator, emulsifier and antibacterial preservative with secondary amine can all catalyze the potential nitrosation reaction at temperatures above 35°C or at acidic pH [34]. In addition, the storage vat with the nitrite for anti-corrosion and some packaging materials with residual toxic solvent might result in the nitrosamines formation. The packaging materials of cosmetics are mainly based on plastic, and simultaneously, N, N-DMA is widely applied in the packaging materials due to high thermal stability, low corrosion, and low toxicity. Thus, the DMA in the plastic containers could volatilize and shift to the contents, resulting in polluting the cosmetics [35].
Detection Methods of Nistrosamines
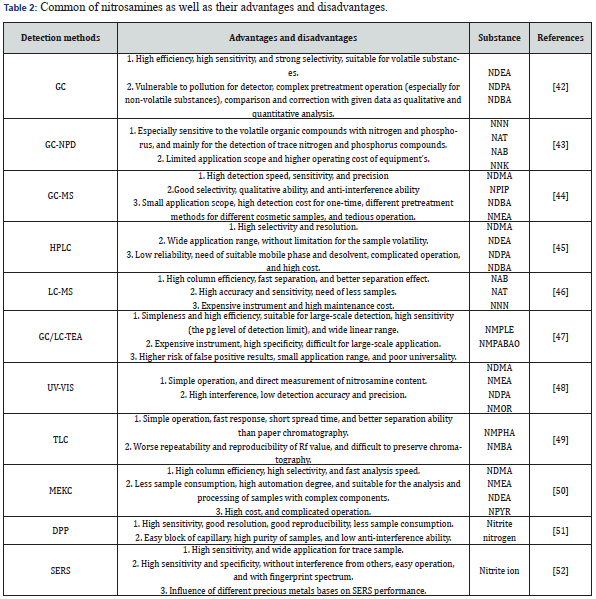
It is greatly difficult to detect the nitrosoamines in cosmetics, attributed to more types, lower content, greater difference in the properties of nitrosamines, and possible false positive disturbance of new nitrosamine formed in the analysis process [36]. At present, the frequently-used detection methods include gas chromatography (GC), liquid chromatography (LC), gas chromatography-mass spectrography (GC-MS), UVVIS spectrophotometry, thin layer chromatography, micellar electrokinetic capillary chromatography (MECC), polarography, surface-enhanced Raman scattering method (SERS), etc. Furthermore, for the detection of volatile nitrosamines in water substrate, nitrogen phosphorous detection (NPD) and nitrogen chemiluminescence detection (NCD) methods are used as the mass spectra (MS) for cost reduction.
GC is a chromatographic separation and analysis method using gas as mobile phase, suitable for qualitative and quantitative analysis for volatile organic compounds. In principle, the gas sample is brought to the chromatographic column by carrier gas. The forces between stationary phase and sample components in column are different, so the components can be separated from each other due to their different outflow time from column. Similarly, the LC takes liquid as the mobile phase, and the components in mixture are separated in dependence on the difference in the affinity of each component for two phases. This method is characterized by high efficiency, high sensitivity, and simple operation.
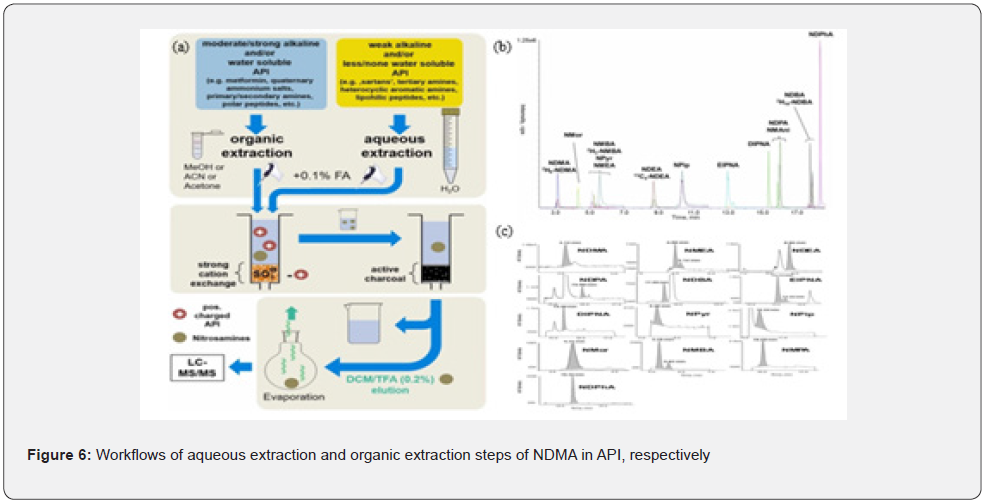
As can be seen by comparison, the GC-MS has not only the separation capability of GC but also the highly sensitive identification capability of MS. GC-MS/MS is used to detect 14 nitrosamines in 44 types of drugs such as sartan and ranitidine. Compared with HPLC, LC-MS has a higher column efficiency and separation speed, withstands higher column pressure and faster flow rate. Thus, LC-MS has the higher peak capacity and separation effect. Small volatile nitrosamines such as NPMA has been detected in active pharmaceutical ingredient (API) by Vogel et al. [37] using LC-MS/MS (Figure 6). (b) Ion chromatograms of all the nitrosamines detected after adding 50 ng/ml aqueous reference solution. (c) Ion chromatograms of quantitative nitrosamines extracted with their own LOD value.
The thermal analysis technology (TEA) is usually used with GC or LC together. The nitrosamines mixture is firstly separated, and then the N-NO bond breaks after through the TEA cracking chamber. The released nitroso (NO) is oxidized by ozone to electronically excited NO2. Accordingly, the decayed radiation intensity is in proportion to the concentration of NO [38]. Because of some advantages such as high sensitivity, high precision and rapid detection, so this method has been widely applied in many fields. The methods mentioned above can qualitatively and quantitatively detect the unknown samples, but they still have some disadvantages of tedious preprocessing process, high detection cost, long detection period, expensive instruments, etc.
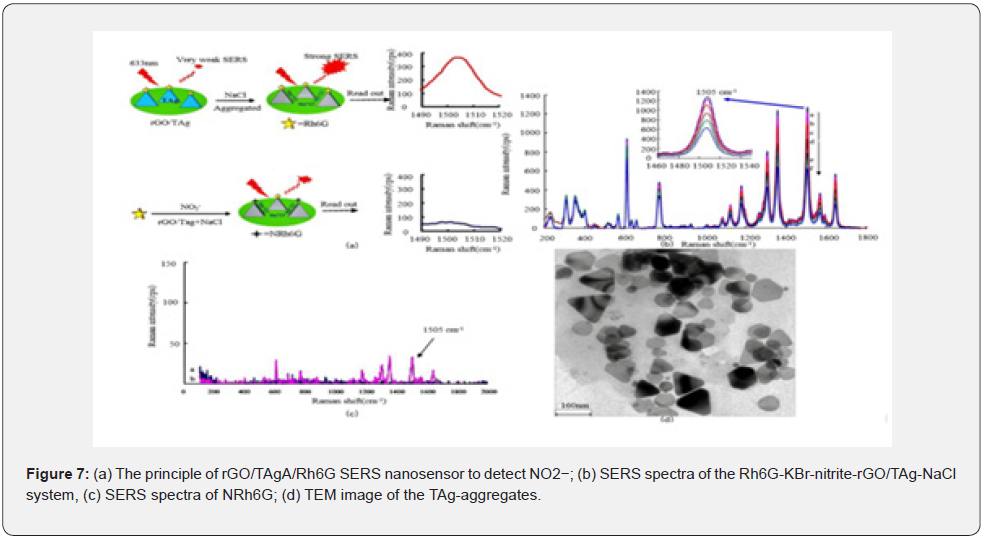
In recent years, Luo et al. used Raman spectra detection based on the surface enhanced Raman scattering (SERS) of precious metals to detect the trace nitrite ions in aqueous solution of nitrosation (Figure 7). This technology can make the signal intensity increase by 108~1012 times, and effectively improve the sensitivity of traditional Raman detection in trace analysis. Moreover, it has advantages such as ease of operation, strong selectivity, freedom from interference of water molecules, and fingerprint spectrum, so with a better detection effect for illegal additives in cosmetics, i.e., nitrosoamines, Rhodamine B, hydroquinone, and crystal violet. Representative detection methods of nitrosamine are listed in Table 2.
Furthermore, there has been a difficulty in directly analyzing the target due to more types of cosmetics and complex components. It is necessary to select an appropriate pretreatment method according to the target to be quantified. The target should be detected after the enrichment of purified samples. At present, the pretreatment technologies of common cosmetic samples include the digestion method, liquid-liquid extraction method, solid phase extraction method, microextraction techonology, microwaveassisted extraction method, ultrasonic-assisted extraction method, etc. [41] added cerium dioxide into porous silicon dioxide (SBA-15). The nitrosoamines are extracted from cosmetic samples with solid-phase microextraction, and meanwhile, the stirring rod is used to improve the adsorption efficiency. This solid-phase microextraction-GC-MS method is very sensitive and efficient in the detection of trace nitrosamines in cosmetics with the stirring rod support base on Ce-SBA-15 development. Miralles et al. [17] detected seven kinds of trace prohibited N-nitrosoamines in cosmetics using reversed phase dispersionliquid- phase microextraction-LC. Accordingly, the results show that the enrichment factor is up to 65, detection limit is 1.8~50 ng/g, with a better repeatability (RSD < 9.8%). More importantly, this method is used to detect different samples of cosmetics, and then obtaining the equal relative recovery (80~113%).
Norms and Standards related to Nitrosoamines in Cosmetics
The GB/T 29669-2013 published in 2014 stipulates the detection methods, detection limits and quantitative limits of ten types of nitrosamines such as NDMA in cosmetics. The industry development, technology progress and cosmetics supervision demand prompt the State Food and Drug Administration to revise the Cosmetic Safety Code, and then the cosmetic safety technical specification (2015) was put forward. A total of 944 kinds of nitrosamines are listed in the prohibited substances table, such as N-nitroso dimethylamine, N-nitroso dipropylamine, and N-Nitrosodiethanolamine. In addition, the standards of the nitrosamines contents in cosmetics are introduced at abroad. For example, the International Organization for Standardization has issued the detection and analysis method for NDELA in cosmetics and proposed the Technical Guide Document. British Standards Institution has published the standard detection method of NDELA in cosmetics. Likewise, France has issued the standard determination method of NDELA in cosmetics. Table 3 lists the norms and standards related to nitrosamines in cosmetics.
Conclusion
With constant improvement of cosmetics performance, their components are more and more complex. Because of longterm contact with skin, the strongly carcinogenic substance in cosmetics such as nitrosoamines could result in greatly potential hazard to the human body. Although some nitrosamine compounds are prohibited in cosmetics in national standards, their coverage and completeness can’t meet the safety supervision demand of cosmetics. Moreover, the nitrosamines detection could be interfered by matrix components of cosmetics, and the existing detection means are insufficient. Therefore, more effort should be devoted to establishing rapid, large-scale, sensitive, accurate and low-cost detection methods for nitrosamines, as well as improving relevant standards and specifications in future. Simultaneously, much attention to the cosmetics’ safety and intensification of industrial supervision would make the studies on nitrosamines contamination in cosmetics develop toward the norms of cosmetics.
Acknowledgments
We would like to acknowledge the financial support from NMPA Key Laboratory of Cosmetic Safety Assessment, Guangdong Institute for Drug Control (KF2021014); Key Laboratory of Technological Innovation of Gungdong Medical Products Administration (2021ZDZ03); Key Laboratory of Green Cleaning Technology & Detergent of Zhejiang Province (Q202204), China Postdoctoral Science Foundation (2021M690068), and Central University Basic Research Fund of China (JUSRP221018, JUSPR622038).
References
- China Oral Care Industry Association (2021) China has become the second largest cosmetics consumer market in the world. Oral Care Industry 31(01): 59.
- Gushgari AJ, Halden RU (2018) Critical review of major sources of human exposure to N-nitrosamines[J]. Chemosphere: Environmental toxicology and risk assessment 210: 1124-1136.
- Hua Du, Jiapeng L, Pengcheng W, Lin Li, Wang Y (2018) Impact of tobacco-specific nitrosamine-derived DNA adducts on the efficiency and fidelity of DNA replication in human cells[J]. The Journal of Biological Chemistry 293(28):11100-11108.
- Hirata N, Yamada S, Sekino Y, Kanda Y (2017) Tobacco nitrosamine NNK increases ALDH-positive cells via ROS-Wnt signaling pathway in A549 human lung cancer cells. The Journal of Toxicological Sciences 42(2):193-204.
- Zheng Xingquan (2000) Current status and monitoring methods of nitrosamines in cosmetics. Journal of Environmental Hygiene 1(3): 32-37.
- Tunick M, Veale HS, Harrington GW (1982) Qualitative detection of N-nitrosodiethanolamine in cosmetic products. Food and Chemical Toxicology: An International Journal Published for the British Industrial Biological Research Association 20(4): 473-474.
- Chou H J, Yates R L, Havery D C, Weninger JA (1995) Determination of 2-ethylhexyl 4-(N-methyl-N-nitrosamino) benzoate in commercial sunscreens and cosmetic products. Journal of Aoac International 78(6): 1378-1383.
- Zhang Ai, Yongmei L, Yun S, Juan LV, Juan Yang (2014) Characterization of pharmaceuticals and personal care products as N-nitrosodimethylamine precursors during disinfection processes using free chlorine and chlorine dioxide[J]. Journal of Hazardous Materials 276: 499-509.
- Lim D S, Roh T H, Kim M K, Chan Kwon Y, Choi SM, et al. (2018) Risk assessment of N-nitrosodiethylamine (NDEA) and N-nitrosodiethanolamine (NDELA) in cosmetics[J]. Journal of Toxicology and Environmental Health, Part A, 81(12): 465-480.
- Choi N R, Kim Y P, JI WON HYUN, et al. Identification and quantification of seven volatile n-nitrosamines in cosmetics using gas chromatography/chemical ionization-mass spectrometry coupled with head space-solid phase microextraction. Talanta: The International Journal of Pure and Applied Analytical Chemistry,2016,14869-74.
- Dong Hao, Guo Xindong, Xian Yanping, Luo H, Wanget Bin, et al. (2015) A salting out-acetonitrile homogeneous extraction coupled with gas chromatography-mass spectrometry method for the simultaneous determination of thirteen N-nitrosamines in skin care cosmeticsJ Chromatogr A 1422: 82-88.
- Braun O, Marlowe P, Bassel S. AMPS and N, N-dimer acrylamide copolymer of the new trans latex. Cosmetic Use: CN200610082747.1. 2006-11-29.
- Fiume M (2017) Isostearamidopropyl Morpholine Lactate. Int J Toxicol 36(Suppl.2): 35S-36S.
- Zhou Zelin, Feng Jun, Li Qin (2011) Investigation on the frequency of cosmetic ingredients. Flavour Fragrance Cosmetics, 2011(6): 29-32.
- Kim SS, Yoon WJ, Hyun CG (2010) Down-regulation of tyrosinase, TRP-2 and MITF expressions by neolitsea aciculata extract in murine B16 F10 melanoma. International Journal of Pharmacology 6(3): 290-295.
- Ma Qiang, Xi Haiwei, Wang Chao, Hua BAI, Guang-Cheng XI, et al. (2011) Determination of 10 volatile nitrosamines in cosmetics by gas chromatography-Tandem[J]. Chinese Journal of Analytical Chemistry 39(8):1201-1207.
- Miralles P, Chisvert A, Salvador A (2018) Determination of N-nitrosamines in cosmetic products by vortex-assisted reversed-phase dispersive liquid-liquid microextraction and liquid chromatography with mass spectrometry. J Sep Sci 41(15): 3143-3151.
- Xian Qiming, Huang Peili (2008) Determination of leachable N-nitrosamines in latex by gas chromatography - mass spectrometry/SIM. Journal of Instrumental Analysis 27(4): 445-447.
- Zhou Qidong (2017) Application of gas chromatography-tandem mass spectrosmetry for the determination of N-nitrosamines in cosmetics. South China University of Technology.
- Tao Jinxiong, Zhang Haixuan, Lin Ziwei (2019) Determination of two N-nitrosamines in rubber materials by gas chromatography-mass spectrosmetry. Chinese Journal of Analysis Laboratory 38(9):1051-1056.
- Fujii T, Ito T (2004) Development of novel nonionic thickening agent and its application in cosmetics. Detergent & Cosmetics 27(8): 8-13.
- He Pingsheng, Li Chune, Chen Ying, et al. Langmuir-Blodgett films of polyimide I. Langmuir- Blodgett films of poly N, N - dimethyloctadecylamine precursor. Chinese Journal of Chemical Physics 1999(4): 476-480.
- Qingdao University. A kind of transparent lipstick and its preparation process, CN201811617292. 82019-03-01.
- Preparation method of beauty cream with the effect of eliminating pouches: CN201611138112.92018-02-09.
- Gettings S D, Dressler W E, Franz T J, et al. (1995) Assessing risk from N-nitrosamines in cosmetic products. Cosmetics & Toiletries 110: 72-80.
- Joo KM, Shin MS, Jung J H, Min Kim Boo, John Whan Lee, et al. (2015) Determination of N-nitrosodiethanolamine, NDELA in cosmetic ingredients and products by mixed mode solid phase extraction and UPLC-tandem mass spectrometry with porous graphitic carbon column through systemic sample pre-cleanup procedure. Talanta: The International Journal of Pure and Applied Analytical Chemistry 137: 109-119.
- Guo Huifang. Detection of nitrosamines in several typical nitrogen-containing surfactants. Shanxi: China Research Institute of Daily Chemical Industry, 2019.
- General Administration of Quality Supervision, Standardization Administration of the People's Republic of China. Determination of 10 volatile nitrosamines such as N-nitrosodimethylamine in cosmetics Gas chromatography-mass spectrometry/mass spectrometry: GB/T 29669-2013. 2013.
- Li Junyuan, Cao Jiang, Han Wei, et al. (2007) Analysis on the structure and performance of cocamidopropyl betaine. Journal of Chemical Industry & Engineering 28(2): 46-50.
- Lim DS, Kin SK, Kwon YC, Kwon YC, Roh TH, et al. (2018) Formation and inhibition of N-nitrosodiethanolamine in cosmetics under pH, temperature, and fluorescent, ultraviolet, and visual light. J Toxicol Environ Health A 81(9): 241-253.
- Xu Fang, Lin Xiaohui (1994) Nitrosation reaction of N, N-dimethylaniline. Journal of Fujian Medical University 28(3): 276-278.
- Choi J, Valentine R L (2002) Formation of N-nitrosodimethylamine (NDMA) from reaction of monochloramine: a new disinfection by-product. Water Research 36(4): 817-824.
- Sun Zhi (2010) Investigation of the N-nitrosamines formation mechanisms based on various amines as precursor[D]. Beijing: Beijing University of Technology.
- Fan Liqin, Yang Xianqing, Chen Shengjun (2009) Influence of light and temperature on degradation of N-nitrosodimethylamine and N-nitrosodiethylamine. South China Fisheries Science 5(3): 53-58.
- Yu Wenjing, Zheng Chen, Zhang Rong, et al. The determination of N, N-dimethylacetamide reidue in cosmetics by gas chromatography/mass spectrometry. Flavour Fragrance Cosmetics, 2013(6): 33-36.
- Wang Haiyan, Li Bin, Sun Lei (2020) Establishment and application of screening platform for high-risk compounds in cosmetics. China Food & Drug Administration Magazine, 2020(05): 72-79.
- Yanghe Luo, Guiqing Wen, Jinchao Dong, et al. SERS detection of trace nitrite ion in aqueous solution based on the nitrosation reaction of rhodamine 6G molecular probe. Sensors and Actuators, B. Chemical,2014,201336-342.
- Kong Xiangyi, Lin Peng, Fang Enhua (2020) Research progress in analysis of nitrosamine in food. Journal of Analytical Science, 36(4): 597-605.
- Yanghe Luo, Guiqing Wen, Jinchao Dong (2014) SERS detection of trace nitrite ion in aqueous solution based on the nitrosation reaction of rhodamine 6G molecular probe. Sensors and Actuators, B. Chemical 2013: 36-342.
- Zhang Yanshu, Lin Zhenhua, Hu Yuling (2016) Advance on pretreatment methods for cosmetic samples analysis. Journal of Instrumental Analysis 35(2): 127-136.
- Alhooshani K (2020) Determination of nitrosamines in skin care cosmetics using Ce-SBA-15 based stir bar-supported micro-solid-phase extraction coupled with gas chromatography mass spectrometry. Arabian Journal of Chemistry 13(1): 2508-2516.
- Liu Shuping, Guan Weiping, Diao Bingxiang (2012) Determination of N-nitrosamines in water by Purge and trap-gas chromatography (PT-GC). Water Purification Technology 31(1): 68-69.
- Lu Hongbing, Zhong Kejun, Mao youan (2004) Research on determination of tobacco-specific nitrosamines in cigarette smoking by GC-NPD. Journal of Hunan City University(Natural Science)13(4): 55-60.
- Chen CX, Wang CH, Zhou HS, et al. (2017) Comparison of gas chromatography-mass spectrometry and gas chromatography-tandem mass spectrometry with electron ionization for determination of N-nitrosamines in environmental water. Chinese Journal of Spectroscopy Laboratory, 2017. 168: 1400-1410.
- Han Ying, Wang Jihe, Li Jun, et al (2017) Simultaneous determination of four kinds of nitrosamines in water by high performance liquid chromatography. China Water & Wastewater 33(06): 131-134.
- Tang LY, Ding SC, Guo ZM (2003) LC-MS/MS improved method of tobacco specific nitrosamines in mainstream smoke. Chemical Research and Application, 2013. 25: 111-116.
- Xing Kunming, Liu Hua, Wang Yikun (2016) GC-TEA determines NDELA in cosmetics. Guangzhou Chemical Industry 44(23): 84-88.
- Comparative study about oxidation of trace N-nitrosamines by seven oxidation processes with a sensitivity improved determination method. Separation and Purification Technology,2020,236116009-1-116009-9.
- Wei Tianjun, Feng Guangying (1981) Thin layer analysis identification of volatile nitrosamines in grains-Secondary unfolding light solution. Chemical Journal of Chinese Universities 2(03): 317-320.
- Bell L, Murray G (2005) Selective photo-reduction of N-nitroamines combined with micellar electrokinetic chromatography and laser fluorimetric detection. Journal of Chromatography. Analytical technologies in the Biomedical and Life Sciences 826(1-2):160-168.
- Luan Xiukun, Gong Weidong (1993) Determination of nitrite nitrogen in drinking water by oscilloscopy polarography. Chinese Journal of Public Health 9(12): 556.
- Yang Hanru. SERS sensor based on surface reaction was developed to detect nitrite ions. Shanghai Normal University, 2018.
- Zhao Tonggang (2007) Cosmetic hygiene practices. Beijing Daily Chemical (3): 27.
- SPGG 20201207002-2020 Safety and Technical Standards for Cosmetics 2015.
- IX-ISO. ISO 10130-2009 Cosmetics Analytical method Nitrosamine: Detection and determination of diethanol-N-nitrosamines in cosmetics with high-performance liquid chromatography (HPLC), post-column photolysis, and derivatization (NDELA)[S]:ISO 10130-2009.
- English, T., FD T75-637:2013-07-17; FD ISO/TR 14735:2013-07-17.
- BS ISO 15819-2014 Cosmetic Analysis Methods Nitrosamines: Use liquid chromatography-mass spectrometry tandem (HPLC-MS-MS) to detect and determine N-nitrosodiethanolamines in cosmetics (NDELA): BS ISO 15819-2014. 2014.
- NF T75-631-2008 Cosmetics Analytical Methods - Nitrosamines: Detection and determination of N-nitrosodiethanolamine (NDELA) in cosmetics by HPLC-MS-MS. 2008.






























