Involvement of Cathepsin K and Cathepsin L2 in the Digestion of Melanosome Complexes in Epidermal Keratinocytes by Fennel Extract
Haifeng Zeng1, Sakura Koizumi1 and Kazuhisa Maeda1,2*
1Bionics Program, Tokyo University of Technology Graduate School, Japan
2School of Bioscience and Biotechnology, Tokyo University of Technology, Japan
Submission: October 14, 2021;Published: October 22, 2021
*Corresponding author: Kazuhisa Maeda, Bionics Program, School of Bioscience and Biotechnology, Tokyo University of Technology Graduate School, Japan
How to cite this article: Haifeng Z, Sakura K, Kazuhisa M. Involvement of Cathepsin K and Cathepsin L2 in the Digestion of Melanosome Complexes in Epidermal Keratinocytes by Fennel Extract. JOJ Dermatol & Cosmet. 2021; 4(3): 555639. DOI: 10.19080/JOJDC.2021.04.555639
Abstract
In healthy skin, melanosome complexes are digested along with keratinization in the epidermal keratinocytes. However, in senile lentigo, large melanosome complexes accumulate in the basal and spinous epidermal layers. Melanosome complexes are degraded by lysosomes in human epidermal keratinocytes. We investigated how fennel extract affects melanosome degradation in melanosome-containing cultured epidermal keratinocytes. We also investigated the potential of fennel extract to increase intracellular lysosomal digestive enzyme activity using immunostaining and gene expression analysis. The fennel extract reduced tyrosinase-related protein (TRP-1), a mature melanosome membrane protein, and increased cathepsin K and cathepsin L2 expression. The intracellular cathepsin and TRP-1 co-localization was observed in melanosome-containing keratinocytes. In a 3D human epidermis model, the fennel extract increased cathepsin K expression, mainly in the basal and spinous epidermal layers, as well as in the entire stratum granulosum and stratum corneum. Cathepsin L2 expression increased mainly in the basal and the spinous epidermal layers. These results suggest that the fennel extract-induced digestive enzymes were involved in melanosome complex membrane protein degradation and keratinization-related melanin dispersion. Moreover, melanosomes could be degraded in epidermal keratinocytes by promoting cathepsin K and cathepsin L2 activities. Therefore, fennel might prove effective in making senile pigmentation fade.
Keywords:Cathepsin; Fennel extract; Lightening; Lysosome; Melanosome digestion; Pigmented spots
Introduction
Epidermal melanin is important in the regulation of skin color and determines the wide variety of skin colors associated with ethnic diversity [1]. In healthy skin, melanosome complexes in the epidermis are digested along with keratinization. However, in senile lentigo, they accumulate as large melanosome complexes in the basal and spinous layers of the epidermis. Thus, the size, number, and distribution of melanosome complexes are the main determinants of skin color. Melanin is synthesized in melanosomes, which are the organelles found in the pigment cells (melanocytes) in the basal layer of the epidermis. In the melanosomes, tyrosine is converted to 3,4-dihydroxyphenylalanine (DOPA), and DOPA to dopaquinone, by the action of the enzyme tyrosinase. After dopaquinone formation, the eumelanin pathway proceeds as follows: first, dopachrome is converted to 5,6-dihydroxy-1H-indole (DHI) by spontaneous decarboxylation or to 5,6-dihydroxyindole-2-carboxylic acid (DHICA) catalyzed by dopachrome tautomerase (DCT) [2]. Melanogenesis can culminate in eumelanin by oxidative polymerization reactions following the formation of DHI and DHICA [2]. Tyrosinase, a melanin synthase, is synthesized separately from melanosomes and transported by vesicles to immature melanosomes. Tyrosinase-related protein-1 (TRP-1) is present in mature melanosomes and has been used as a marker for melanosomes [3,4]. The melanin synthesized near the nucleus of melanocytes accumulates in melanosomes, and the mature melanosomes are transported to the vicinity of the cell membrane via long-range bidirectional microtubules and directly under the cell membrane via short-range unidirectional actin fibers. Subsequently, it combines with the cell membrane, is transferred to the adjacent keratinocytes, and accumulates on the nucleus of the keratinocytes to form a nuclear cap (melanin cap) [5,6]. We also reported in an earlier study that large melanosome complexes accumulate in the keratinocytes of the basal and spinous layers [6], resulting in senile pigmented lesions.
Melanosome complexes are known to be degraded by lysosomes in normal human epidermal keratinocytes [7]. It has been reported that aspartic proteases and cysteine proteases are involved in the promotion of tyrosinase degradation in acidic melanosomes [8]. Cysteine proteases are a type of acidic protease, and cathepsin L2 is considered to act as a cysteine protease in the degradation of tyrosinase in melanosomes. Studies on racial and skin color differences have reported that melanosomes of lightskinned keratinocytes are degraded faster than those of darkskinned keratinocytes [9] and the activity of the enzyme cathepsin L2 is reported to be higher in light-skinned keratinocytes than in dark-skinned keratinocytes [10]. Because cathepsin K is also a cysteine protease, it is thought to have an effect of degrading tyrosinase in melanosomes. In Japan, medicated whitening cosmetics (“quasi-drugs”) that inhibit tyrosinase activity, such as kojic acid, arbutin [11], and rucinol, promote the degradation of tyrosinase, such as linoleic acid [12]. In addition, stable vitamin C derivatives, vitamin C ethyl with antioxidant properties [13], and anti-inflammatory properties, such as that in tranexamic acid [14], are being researched and developed [15]. However, agents such as rhododendrol and magnolignan are currently not used because they release hydroxyl radicals from tyrosinase, are highly cytotoxic to melanocytes, and may induce leukoderma [16]. The use of such substances, wherein hydroxyl radicals are generated by tyrosinase to regulate melanin production, poses a risk of causing vitiligo. Therefore, there is a growing need for developing skin–¬whitening ingredients with a mechanism that promotes the degradation of melanosome complexes accumulated in keratinocytes.
Fennel (scientific name: Foeniculum vulgare Mill.) is a perennial herb belonging to the Apiaceae (Umbelliferae) family. The young leaves and seeds (fennel seeds) have a sweet aroma and bitter taste, and effectively promote digestion and deodorizing. Since ancient times, fennel has been used as a spice and herb for food and medicinal purposes. The constituents of fennel seed oil are (E)-anethole, limonene, methyl chavicol, fenchone, α-pinene, and (Z)-β-ocimene [17,18]. Fennel seed oil reportedly exhibits anti-inflammatory properties [17]. In addition, fennel seed oil and seed extract in methanol or ethanol reportedly display antioxidant properties (DPPH scavenging activity, ferric reducing activity, and inhibition of peroxidation of linoleic acid) [19-21]. The composition of fennel seed methanolic extracts was characterized by its richness in quinic acid, 4-O-caffeoylquinic acid, p-coumaric acid, and 4-O-caffeoylquinic acid [20]. The water extract of fennel fruits also reportedly exhibits strong antioxidant activity (inhibition of peroxidation of linoleic acid) [21-23], and 4-O-β-D-glucosyl sinapyl alcohol, 4, 9-di-O-β -glucosyl alcohol, and 4-β-glucosyloxy benzoic acid were identified as its active components [23]. Based on this background, we investigated the degradation of melanosome complexes in cultured human keratinocytes by fennel extract, a natural component with reportedly strong antioxidant properties and analyzed the mechanism by focusing on lysosomes effects.
Materials and Methods
Materials
Dried fennel fruit (Foeniculum vulgare Miller fruits; Place of origin: China), sold as a crude drug, was purchased from Nakaya Hikojuro Pharmacy Co. Ltd. (Ishikawa, Japan). Anti-tyrosinaserelated protein-1 (TRP-1) antibody, TMH-2 [24], was used. Anticathepsin K and anti-cathepsin L2 antibodies were purchased from BioVendor Laboratory Medicine, Inc. (Brno, Czech Republic) and Abcam plc. (Cambridge, United Kingdom). Alexa Fluor 488 goat anti-mouse IgG antibody, Alexa Fluor 488 goat anti-rabbit IgG antibody, Alexa Fluor 546 goat anti-rabbit IgG antibody, Alexa Fluor 546 goat anti-rat lgG antibody, Alexa Fluor 647 goat antirat IgG antibody, Lyso Tracker Green DND-26, Lyso Tracker RED DND-99, and Hoechst 33342 Nuclear Stain were purchased from Thermo Fisher Scientific (Waltham, MA, USA). The other reagents used in the experiments were purchased from Wako Pure Chemical Industries (Osaka, Japan). HaCaT cells and B16 melanoma cells were purchased from Cell Lines Service GmbH (Eppelheim, Germany). Human epidermal 3D models were purchased from Japan Tissue Engineering Co., Ltd (Aichi, Japan).
Fennel extract preparation
Thirty grams of fennel was soaked in 300 mL of 0%, 25%, 50%, 75%, and 99.5% ethanol solution for one week. The solvent was removed using a centrifugal concentrator (Savant SpeedVac Concentrators, Thermo Fisher Scientific, Inc., Waltham, MA, USA) and Bio Freeze Dryer (BFD-6F2, Nihon Freezer Co., Ltd., Tokyo, Japan); then, the yield was determined. The inhibition of L-DOPA autoxidation and the promotion of melanosome degradation in keratinocytes were measured as follows: First, L-DOPA autoxidation was measured by adding 90 μL of a PBS solution (pH 7.4) of 10 mmol/L L-DOPA to the wells of a 96-well microplate. Then, 10 μL of fennel extract was added with concentrations of 0 (control), 1.25, 2.5, and 5 mg/mL and incubated at 37°C for 2 days. The change in absorption at 550 nm was measured and used to quantify the L-DOPA autoxidation relative to the control. Second, for measuring the melanosome degradation-promoting effect, 20,000 cells/99 μL of melanosome-containing and melanosomefree HaCaT cells were seeded in the wells of a 96-well microplate. Then, 1 μL of fennel extract was added with concentrations of 0 (control), 1.25, 2.5, and 5 mg/mL and incubated at 37°C for 4 days. The absorption at 550 nm was measured and used to calculate the ratio of melanosome-containing to melanosome-free HaCaT cells. The absorption value for each fennel extract trial relative to the control was calculated; this value represents the extent of degradation in melanosome-containing keratinocytes. Both experiments were conducted with n = 3.
Effect of Fennel Extract on the Proliferation of HaCaT Cells and B16 Melanoma Cells
A fennel extract solution of 10 mg/mL was prepared by adding 1 mL of dimethyl sulfoxide (DMSO) to 10 mg of fennel extract and diluted to 5 mg/mL with DMSO. Cells cultured in 10% FBSDMEM (Fetal Bovine Serum-Dulbecco’s Modified Eagle Medium) at 200,000 cells/mL, seeded in 96-well plates (AGC Techno Glass Co., Ltd., Shizuoka, Japan) at 99 μL/well, and cultured for 1 day. Then, 1 μL of the control (DMSO) and fennel extract were added and cultured in 2% FBS-DMEM medium for 2 days. Cell Counting Kit-8 (Dojindo Laboratories, Kumamoto, Japan) was added at 10 μL/well and cultured for 2 h, and the absorbance at 450 nm was measured with a microplate reader (Power Scan HT, BioTek, Winooski, VT, USA) and used as a measure of the cell count. In this method, WST-8 [2-(2-methoxy-4-nitrophenyl)-3-(4- nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium sodium salt)], which produces highly sensitive water-soluble formazan, was used. The number of living cells was determined by measuring the absorbance at 450 nm of the water-soluble formazan produced after reduction by intracellular dehydrogenase. There is a linear proportional relationship between the number of cells and the amount of formazan produced. The experiment was repeated three times (n=3).
Melanosomal Lysis and Absorbance Measurement
The medium in the 100 mm dish to which control, 15 μg/mL and, 30 μg/mL of fennel extract and 15 μmol/L of chloroquine had been added was removed. Trypsin-EDTA (10 mL) was added and left in a 5% CO2 incubator for 30 min, and the cells were collected in a 15 mL centrifuge tube and counted. The medium in the centrifuge tube was removed, the cell count was measured, and 1 mol/L NaOH was added according to the number of cells. In a 96- well plate, 100 μL of each sample was added and the absorption spectra from 400 nm to 700 nm and the value at 550 nm were measured.
Effect of Fennel Extract on Cathepsin K and Cathepsin L2 in Keratinocytes
A solution of 4 mmol/L acetate buffer (pH 5.6), phosphatebuffered saline (PBS; pH7.4), 1 mmol/L cathepsin K substrate, 0.1 mmol/L cathepsin L substrate, and cathepsin B inhibitor solution was prepared. Cathepsin L2 specifically degrades the substrate Z-Phe-Arg-AMC and releases the fluorescent substrate 4-methylcumaryl- 7-amide (AMC) [25,26]. The reaction equation is shown in Equation (1).
Z-Phe-Arg-AMC → Z-Phe-Arg + AMC (1)
The fluorescence intensity of AMC was detected using a fluorescence microplate reader (Power Scan HT) at 37°C. As Z-Phe- Arg-AMC is also a substrate of cathepsin B, cathepsin L2 activity was specifically measured by mixing the reaction solution with [L-3-trans-(propylcarbamoyl)oxirane-2-carbonyl]-L-isoleucyl-Lproline (CA-074), a specific inhibitor of cathepsin B [27]. The cells were prepared at 200,000 cells/mL, and seeded at 99 μL/well in 96-well plates, and cultured for 1 day. After 3 days of culture, the medium on the 96-well plate was removed and discarded. The cell plates were washed twice with PBS, and frozen. After thawing, (1) 10 μL of 1 mmol/L cathepsin K substrate and 90 μL of PBS buffer were added to each well, or (2) 10 μL of 0.1 mmol/L cathepsin L2 substrate, 10 μL of cathepsin B inhibitor solution, and 80 μL of 4 mmol/L acetate buffer (pH 5.6) were added to each well. The fluorescence intensity (excitation, 360 nm, emission, 460 nm) was measured with a microplate reader, and the plate was placed in an incubator at 37°C for 4 h. The activities of cathepsin K and cathepsin L2 were measured over 4 h. The experiment was repeated three times (n=3).
Effect of Fennel Extract on mRNA Expressions of Cathepsin K and Cathepsin L2
HaCaT cells were seeded in 35 mm dishes at 0.999 mL/dish (30,000 cells/mL) and cultured for 1 day. Fennel extract was dissolved in DMSO to prepare solutions of 25 mg/mL and 50 mg/ mL, and 1 μL of extract was added to each dishes and cultured for 3 days (n=3). RNA was extracted from the cultured cells using an RNA extraction kit (RNeasy Mini Kit). A real-time PCR system was used to measure the relative mRNA expression levels of CTSK, CTSL2, and GAPDH. Primers were obtained from Qiagen N.V. (Venlo, The Netherlands).
Confocal Laser Scanning Microscopy Analysis of the Effects of Fennel Extract on Lysosomes and TRP-1 in Melanosome-Containing Keratinocytes
HaCaT cell suspensions (70,000 cells/mL) containing melanosomes were prepared, and 1 mL of the suspension was added to each chamber slide and cultured for 1 day. The fennel extract was dissolved in DMSO to prepare solutions of 6.25, 12.5, and 25 mg/mL, 1 μL was added per chamber and cultured for 3 days. The chamber slides were washed with serum-free DMEM, and fluorescence reagent (Lyso Tracker Green DND-26 diluted 60,000-fold in 10% FBS-DMEM medium) was added (500 μL per slide). After collecting the fluorescence reagent and washing with PBS, 500 μL of 4% PFA was added for 10 min at 4°C to fix the cells. After fixation, the cells were washed with PBS and incubated with 100 μL of 10% goat serum-PBS solution for 60 min. The cells were washed five times with PBS and then permeabilized with 0.1% Triton X-100 for 30 min. The cells were washed four times with PBS solution. The primary antibody (anti-TRP-1 antibody) was added and the cells were incubated for 2 h at room temperature. The cells were washed three times with PBS, and then 2000-fold diluted Alexa Fluor 546 goat anti-rat IgG antibody was added for 1 h at room temperature. The nuclei of the cells were stained and observed with the confocal laser scanning microscope.
Confocal Laser Scanning Microscopy Analysis of the Effects of Fennel Extract on Lysosomes and Cathepsin L2 in Melanosome-Containing Keratinocytes
Melanosome-incorporated cells were added to chamber slides at 500 μL/well (100,000 cells/mL) and cultured for 2 days. Then, 5 μL of solution was added so that the final concentrations of fennel extract and chloroquine were 15 μg/mL, 30 μg/mL, and 15 μmol/L, and the cells were cultured for 2 days. As a control, 5 μL of DMSO was added. The chamber slides were washed with serum-free DMEM, and the fluorescence reagent (LysoTracker Green DND-99 diluted 60,000 times in 10% FBS-DMEM medium) was prepared and added to the slides (500 μL per slide), and cultured for 60 min. After removing the fluorescence reagent, the slides were washed with PBS solution, and 500 μL of 4% paraformaldehyde (PFA) in phosphate buffer was added for 10 min at 4°C to fix the cells. After fixation, the cells were washed with PBS and incubated with 100 μL of 10% goat serum-PBS solution for 60 min. The cells were washed five times with PBS solution and permeabilized with 0.1% Triton X-100 for 5 min. The cells were washed four times with PBS solution. The primary antibody (anti-cathepsin L2 antibody) was then added, and the cells were incubated for 2 h at room temperature. The cells were washed with PBS solution three times, then Alexa Fluor 546 goat anti-rabbit IgG antibody (1:2000 dilution) was added for 1 h at room temperature. The stained cells were examined under a fluorescence microscope (FV3000, Olympus, Tokyo, Japan).
Cathepsin K, Cathepsin L2, and TRP-1 Staining in Normal Human Epidermal Keratinocytes (NHEKs)
Normal human epidermal keratinocytes (NHEKs, Kurabo Industries Ltd., Osaka, Japan) were cultured in a 75 cm2 tissue culture flask in a CO2 incubator with HuMedia-KG2 (Kurabo), a serum-free medium for tissue culture. After the culture medium was discarded, and melanosomes suspended in the medium were added, the NHEKs were cultured for 1 day to allow melanosomes incorporation. The medium used for the melanosome-incorporated NHEKs was discarded, and the cells were incubated with trypsin/ EDTA solution for 10 min in a CO2 incubator. The cells were then seeded into 35 mm dishes with 1 mL of trypsin/EDTA solution and incubated in the CO2 incubator for 1 day. The cells were incubated in the CO2 incubator for 2 days, and then the final concentrations of fennel extract were 15 μg/mL and 30 μg/mL. DMSO was added as a control. Staining for cathepsin L2, cathepsin K, and TRP-1 was performed as described in previous sections. Cathepsin K was stained used by anti-cathepsin K mouse monoclonal antibody (Santa Cruz Biotechnology Inc. Dallas, TX, USA). Alexa Fluor 647 goat anti-rat IgG antibody was used as the secondary antibody for TRP-1 staining.
Immunohistochemical Analysis of Fennel Extract on the Expression of Cathepsin K andCathepsin L in the 3D models of Human Epidermis
The human epidermis models were cultured in a dedicated medium in the presence (12.5, 25, and 50 μg/mL) and absence of fennel extract for 5 days, then embedded in a Tissue-Tek optimum cutting temperature compound (Sakura Finetek Japan Co., Ltd., Tokyo, Japan). The sections were incubated in 4% paraformaldehyde (PFA) in phosphate buffer (pH 7.4) for 10 min at 4°C, washed three times with PBS, then incubated with 10% goat serum in PBS for 1 h at room temperature in a humid chamber. After three washes with PBS, the sections were incubated overnight at 4°C with anti-cathepsin K and anti-cathepsin L2 antibodies diluted 500 × in PBS. The sections were washed three times with PBS supplemented with 0.05% Tween 20, then incubated with Alexa Fluor 488 goat anti-mouse IgG antibody (1:2000 dilution) or Alexa Fluor 488 goat anti-rabbit IgG antibody (1:2000 dilution) at 37°C for 30 min in the humid chamber. Following one wash with PBS, the sections were mounted onto slides using Fluoromount (Japan Tanner Corporation, Osaka, Japan), covered with a cover glass, and observed under a fluorescent microscope (BX51, Olympus, Tokyo, Japan). The fluorescence intensity and fluorescence area of three tissue sections were quantified using ImageJ (NIH, Bethesda, MD, USA). The fluorescent area/total area × 100 was used as the area ratio, and the results of the area ratio × fluorescence intensity calculation were compared.
Statistical Analysis
Measurements were analyzed for significant differences using the paired t-test function (two-sided) in Microsoft Excel. A P value of < 0.05 was considered statistically significant.
Results
Fennel extraction, L-DOPA autoxidation, and melanosome degradation
The results are summarized in Table 1. The highest extract yield was obtained using 99.5% ethanol and was in the form of an oil. The extract obtained from 75% ethanol was in the form of a plaque, whereas the extracts from 50%, 25%, and 0% ethanol were in the form of dry powders. The autoxidation of L-DOPA was significantly inhibited by the extracts from the 50% ethanol solution; however, it was promoted by the extracts from the 0% ethanol solution. The extracts from the 50% ethanol solution had the strongest effect on melanosome degradation in melanosomecontaining keratinocytes. Subsequent experiments were carried out using fennel extracts from the 50% ethanol solution.
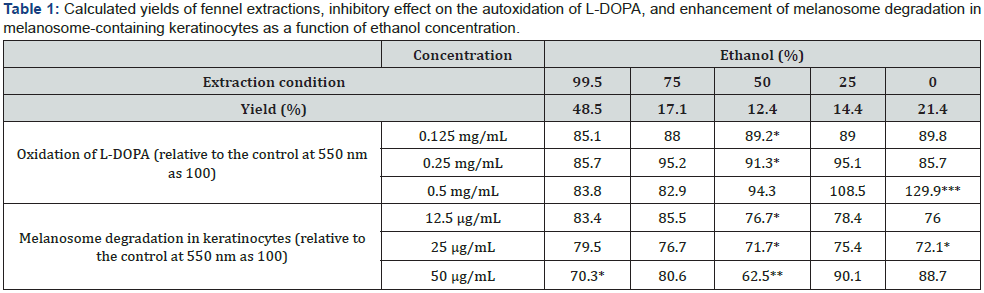
Effect of Fennel Extract on the Proliferation of HaCaT Cells and B16 Melanoma Cells
After 2 days of culture, the fennel extract had no statistically significant effect on the proliferation of B16 melanoma cells or HaCaT cells at any concentration added (25, 50, and 100 μg/mL) and had no effect on cell proliferation at concentrations below 100 μg/mL (Figure 1(a) & (b)).
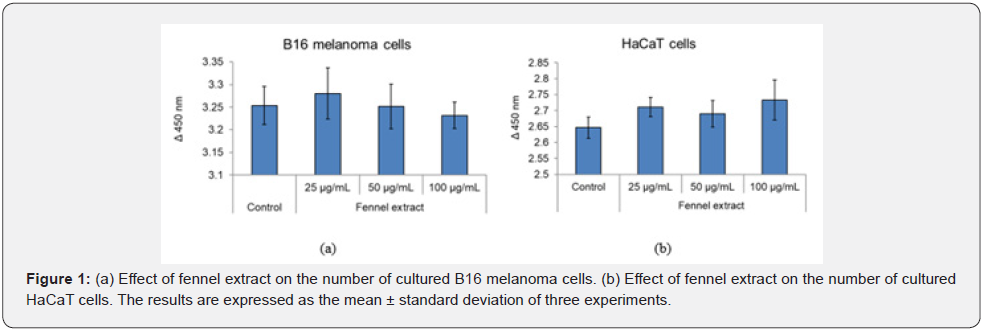
Effect of Addition of Fennel Extract and Chloroquine on the Absorbance of Melanin in Cultured Melanosome- Containing Keratinocytes
The absorption spectra of the cells lysed in 1 mol/L NaOH and the values at 550 nm are shown in Figure 2. Compared with the control, the absorbance was lower in the presence of 15 μg/mL and 30 μg/mL of fennel extract, and higher in the presence of 15 μM chloroquine. Compared with the control, the absorbance at 550 nm was lower in the presence of fennel extract and higher in the presence of chloroquine (Figure 2).
Source: The oxidation and degradation results are expressed as the ratio of the absorbance value at 550 nm of each solvent extract to that of the control, and averaged across three trials. The p-values are indicated as *p < 0.05 vs. control, **p < 0.01 vs. control, ***p < 0.001 vs. control.
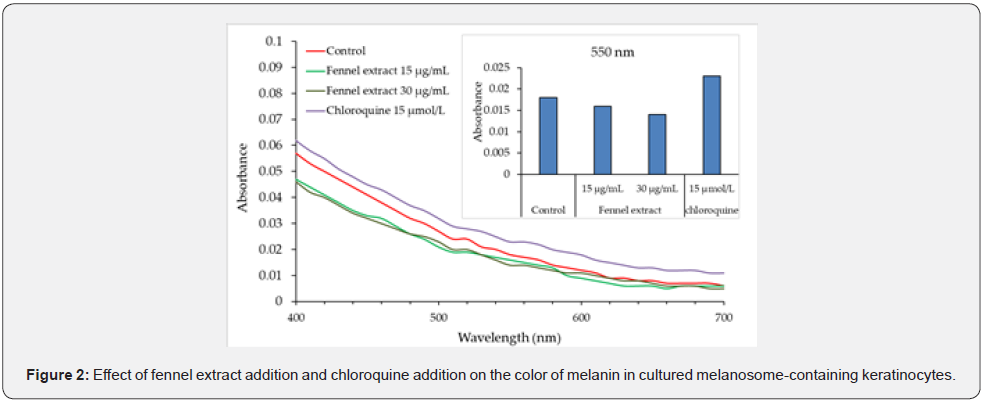
Intracellular Localization of Cathepsin K and Cathepsin L
The intracellular localization of cathepsin K and cathepsin L was examined using immunofluorescent labeling. The localization of cathepsin K and lysosomes was consistent, but cathepsin L was found to exist in the cytoplasmic vesicles as well as lysosomes (Figure 3).
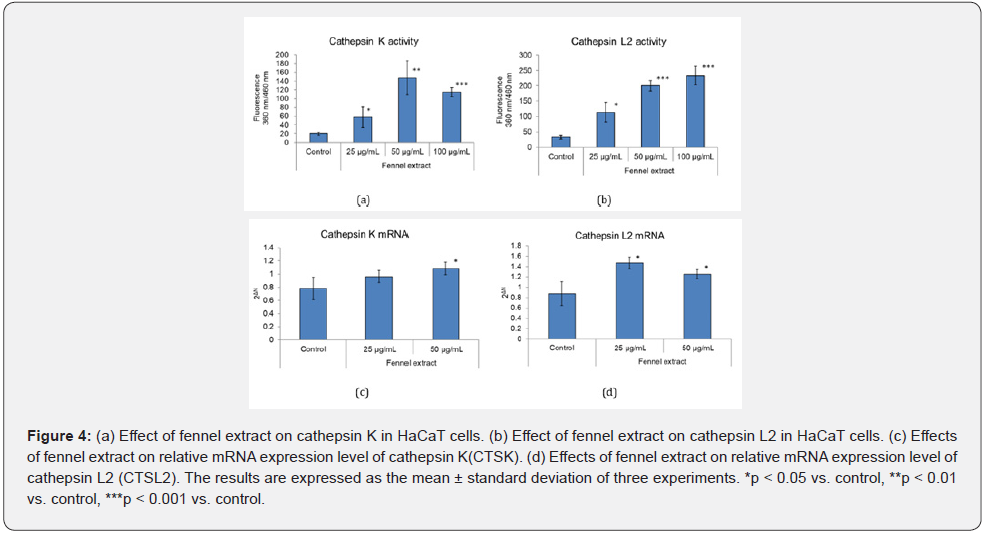
Effects of Fennel Extract on Cathepsin K and Cathepsin L2 in Keratinocytes
The effect of fennel extract on the activities of cathepsin K and cathepsin L2 in cultured keratinocytes was investigated at 25, 50, and 100 μg/mL, and it was found that the activities of these digestive enzymes were promoted higher than 25 μg/mL (Figure 4 (a) & (b)). We also examined the effect of fennel extract on the mRNA expression of cathepsin K, cathepsin L2 in cultured keratinocytes. At 50 μg/mL, the fennel extract increased the expression of CTSK mRNA. The expression of CTSL2 mRNA was increased by 25 μg/mL and 50 μg/mL of fennel extract (Figure 4(c) & (d)).
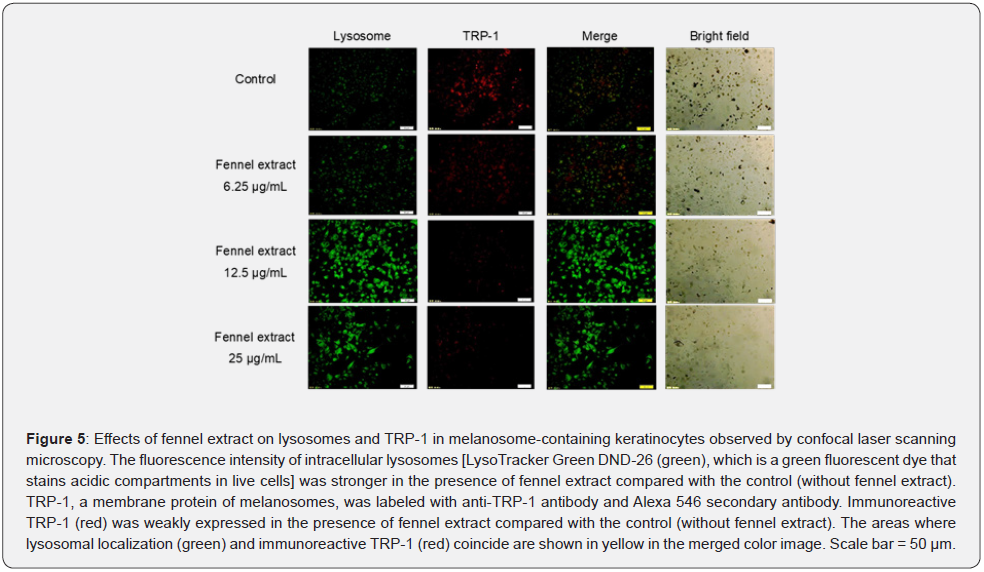
Effects of Fennel Extract on Lysosomes and TRP-1 of Melanosome-Containing Keratinocytes Observed by Confocal Laser Scanning Microscopy
As it was found that fennel extract promoted the activity of intracellular cathepsin K, cathepsin L2, and the relative expression levels of human CTSK mRNA and CTSL2 mRNA, the effects of fennel extract on lysosomes and TRP-1 in melanosomecontaining keratinocytes were investigated using confocal laser scanning microscopy. The effects of fennel extract on lysosome and TRP-1 were visualized using immunostaining. We found that the fluorescence intensity of intracellular lysosomes (green) was stronger in the presence of fennel extract was added compared with the control solution (without fennel extract). Furthermore, the fluorescence intensity (red) of TRP-1 was weaker in cells in the presence of fennel extract (12.5, 25 μg/mL) compared with the control solution (without fennel extract). The fennel extract was found to promote the activity of lysosomes in the degradation of melanosomes, especially at concentrations of 12.5 μg/mL and 25 μg/mL (Figure 5).
Effects of Fennel Extract and Chloroquine on Cathepsin L2 in Melanosome-Containing Keratinocytes
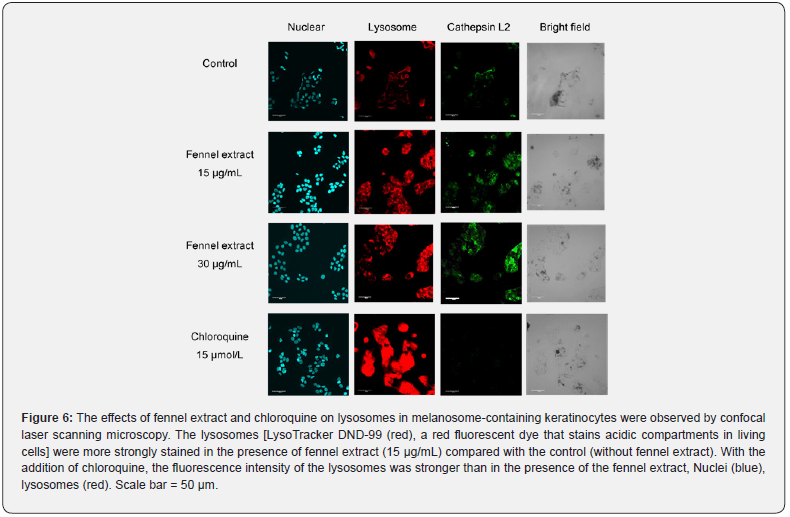
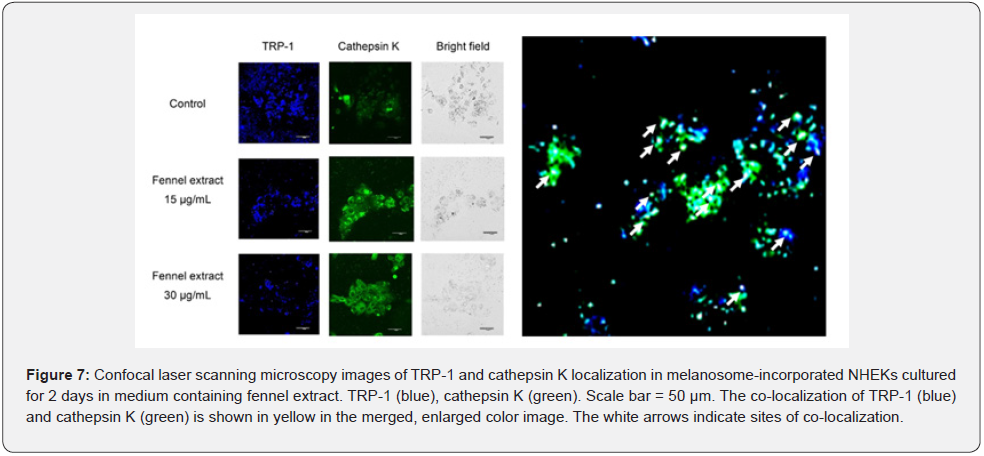
We observed that 15 μg/mL and 30 μg/mL of fennel extract resulted in stronger fluorescence intensity of lysosomes and cathepsin L2 compared with the control (Figure 6). The addition of 15 μmol/L of chloroquine resulted in significantly stronger fluorescence intensity of lysosomes than the addition of fennel extract (Figure 6). However, chloroquine markedly reduced the fluorescence intensity of cathepsin L2 (Figure 6). TRP-1 and cathepsin K in melanosome-incorporated NHEKs were stained with anti-TRP-1 and anti-cathepsin K antibodies after culture in medium containing fennel extract for 2 days. The results of observation by confocal scanning laser microscopy are shown in Figure 7. The fluorescence intensity of TRP-1 was lower after treatment with 15 μg/mL and 30 μg/mL of fennel extract compared with the control. The fluorescence intensity of cathepsin K was increased after treatment with 15 μg/mL and 30 μg/mL of fennel extract compared with the control. The merged images of TRP-1 and cathepsin K showed that there was stronger staining of cathepsin K in the vicinity of the TRP-1 staining after treatment with 15 μg/mL and 30 μg/mL of fennel extract compared with the control. In the bright-field images, there were fewer melanosomes compared with the control. The co-localization of TRP-1 (blue) and cathepsin K (green) is shown in yellow in the merged, enlarged color image. The localization of TRP-1 was found to be consistent with that of cathepsin K in many places.
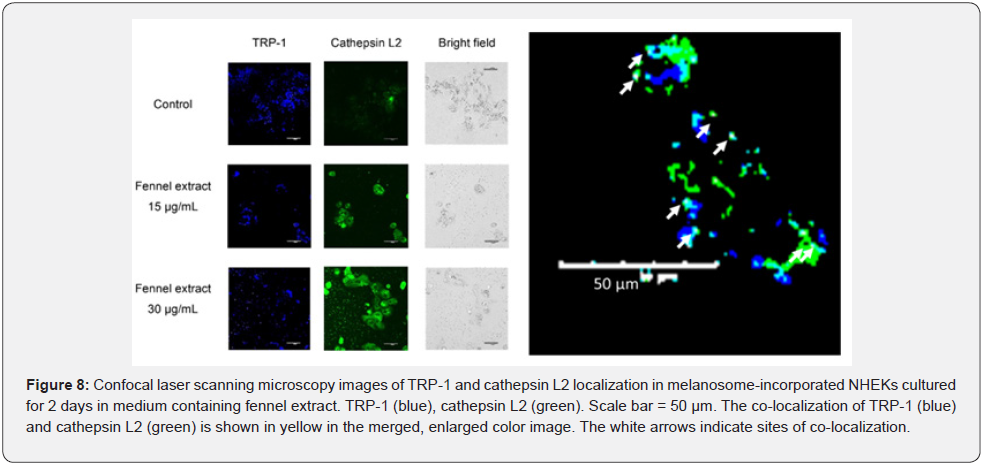
TRP-1 and cathepsin L2 of melanosome-incorporated NHEKs were stained with anti-TRP-1 and anti-cathepsin L2 antibodies, respectively, and observed by confocal scanning laser microscopy when they were cultured in medium containing fennel extract for 2 days. The results are shown in Figure 8. In the presence of fennel extract 15 μg/mL and 30 μg/mL, the fluorescence intensity of TRP- 1 was lower than that of the control. The fluorescence intensity of cathepsin L2 was higher in the presence of 15 μg/mL and 30 μg/mL of fennel extract compared with the control. The merged images of TRP-1 and cathepsin L2 showed that the staining intensity of cathepsin L2 near the TRP-1 staining was stronger in the presence of 15 μg/mL and 30 μg/mL of fennel extract compared with the control. The bright-field images showed that there were fewer melanosomes in the presence of 15 μg/mL and 30 μg/mL fennel extract compared with the control. The co-localization of TRP-1 (blue) and cathepsin L2 (green) is shown in yellow in the merged, enlarged color image. There were also a few cases where the localization of TRP-1 coincided with that of cathepsin L2.
Effect of Fennel Extract on Cathepsin K and Cathepsin L2 Protein Levels in Human Epidermis 3D Models
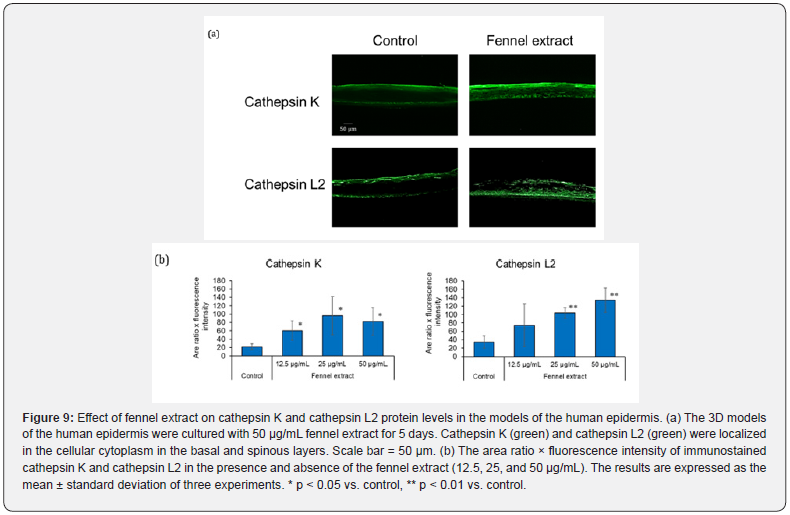
The effects of fennel extract on cathepsin K and cathepsin L2 protein levels in human epidermis 3D models were investigated through an immunohistochemical analysis. In the absence of fennel extract (control), cathepsin K and cathepsin L2 were localized predominantly in the basal and stratum corneum layers of the epidermis (Figure 9(a)). In the 3D human epidermis models cultured in the presence of fennel extract, cathepsin K was localized in the cellular cytoplasm in the basal and spinous layers and throughout the entire stratum granulosum and stratum corneum layers of the epidermis with higher protein level than in the control (Figure 9(a)). Cathepsin L2 was localized in the cellular cytoplasm in the basal and the spinous layers of the epidermis in the presence of fennel extract, and the protein level was higher than that in the control (Figure 9 (a)). The imaging analysis revealed that immunoreactive cathepsin K was significantly increased in the presence of 12.5, 25, and 50 μg/mL fennel extract compared to the control (Figure 9 (b)). Immunoreactive cathepsin L2 was also significantly increased in the presence of 25 and 50 μg/mL fennel extract compared to the control (Figure 9(b)).
Discussion
The addition of fennel extract decreased the amount of melanin in cultured melanosome-containing keratinocytes compared with the control conditions (the absence of fennel extract). The protein level of TRP-1 present in the mature melanosomes was also reduced, suggesting that fennel extract promoted the degradation of the melanosome complex in keratinocytes. In the melanosome-containing keratinocytes, the lysosomal staining intensity was enhanced by the addition of fennel extract. In addition, the amount of cathepsin L2 protein was increased and the intensity of fluorescent antibody staining was also enhanced. Therefore, it is thought that the extract activates lysosomes and cathepsin L2. It has been reported that cathepsin L2 is present close to melanosomes that have transferred to keratinocytes and is involved in the degradation of the melanosome complex. In melanosome-containing keratinocytes, the addition of 15 μmol/L chloroquine resulted in stronger fluorescence intensity and a wider staining area of lysosomes compared with the control and the addition of fennel extract. However, the amount of melanin in the keratinocytes with melanosomes were incorporated was higher in the presence of chloroquine than in the control. Chloroquine is a lysosomotropic drug that raises intralysosomal pH [28] and impairs autophagic protein degradation [29]. Similarly, bafilomycin A1 has the effect of neutralizing the pH of acidic organelles, such as lysosomes, and it has been confirmed that bafilomycin A1 increases the amount of melanin [8]. The amount of melanin in melanosome-containing keratinocytes was higher than in the control in the presence of chloroquine, which may be due to the fact that chloroquine inhibited the degradation of melanosomes incorporated into keratinocytes or acidified lysosomes. Therefore, we hypothesized that melanosomes were degraded by the fusion of melanosomes and lysosomes, and further by the increased expressions of cathepsin K in lysosomes and cathepsin L2 in lysosomes and cytoplasm.
In melanosome-containing keratinocytes, as the staining intensity of the lysosomes was increased by the presence of the fennel extract. Studies on racial and skin color differences have reported that melanosomes from light-skinned keratinocytes are degraded faster than those from darker skin [9] and that the activity of the lysosomal enzyme cathepsin L2 is higher in lightskinned keratinocytes than in dark-skinned keratinocytes [10]. The effect of fennel extract on the activity of the intracellular digestive enzyme cathepsin K and cathepsin L2 was investigated, and it was found that fennel extract promoted the activities of cathepsin K and cathepsin L2. Therefore, the fennel extract may be effective in promoting the degradation of melanosomes in epidermal keratinocytes [8]. We also observed that the fennel extract increased the expressions of cathepsin K and cathepsin L mRNAs, which are the digestive enzymes of lysosomes. The inactive cathepsin precursor is synthesized in the endoplasmic reticulum and then transported to the Golgi apparatus, where it is further glycosylated and phosphorylated to form mannose-6- phosphate protein. Finally, the modified protein is recognized by the mannose-6-phosphate receptor in the lysosome, where the proenzyme is hydrolyzed at low pH and the prodomain is removed to produce active, mature cathepsin [30]. Therefore, the degree of increase in mRNA of cathepsin K and cathepsin L2 by fennel extract may have been lower than the degree of increase in their activity.
Furthermore, we observed the effect of fennel extract on the degradation of melanosomes in epidermal keratinocytes by confocal laser scanning microscopy, and confirmed that fennel extract reduced tyrosinase-related protein-1 (TRP-1), a melanosomal membrane protein. The localization of TRP-1 was found to be consistent with that of cathepsin K in many places. There were also a few cases where the localization of TRP-1 coincided with that of cathepsin L2. From these results, it was confirmed that fennel extract promoted the degradation of melanosomes in epidermal keratinocytes. In addition, cathepsin K and cathepsin L were thought to be involved in the degradation of melanosomes to some extent. Melanosomes phagocytosed by keratinocytes are present in keratinocytes as melanosome complexes. However, the membrane proteins are degraded through keratinization into complexes smaller than 400 nm and dispersed to become invisible. Nevertheless, in senile pigmented spots, multiple melanosome complexes remain in the basal and spinous layers [6]. In this study, applying a human epidermal 3D model, fennel extract increased the expression of cathepsin K mainly in the stratum granulosum and stratum corneum as well as in the basal layer and increased cathepsin L2 expression mainly in the spinous and basal layers. Therefore, these digestive enzymes might be involved in membrane protein degradation in the melanosome complexes and melanin dispersion by keratinization.
Based on the results herein, we believe that fennel extract can degrade melanosomes in epidermal keratinocytes by promoting the digestive enzymes, cathepsin K and cathepsin L2, and this is expected to be effective in helping senile pigmented spots fade.
Conclusion
Melanin amount and TRP-1 expression in mature melanosomes in melanosome-containing keratinocytes were decreased by the addition of fennel extract. Furthermore, cathepsin K and cathepsin L2 immunostaining intensity, activity, and mRNA expression increased. These results suggest that the fennel extract promoted melanosome degradation through the activation of the lysosomal digestive enzymes, cathepsin K and cathepsin L2. The intracellular co-localization of these cathepsins and TRP-1 was observed in melanosome-containing keratinocytes. In the 3D models of the human epidermis, cathepsin K expression was increased by the fennel extract, primarily in the stratum granulosum and stratum corneum but also in the basal layer, and cathepsin L2 expression was increased primarily in the spinous and basal layers. These results indicate that fennel extract can be effective in making senile pigmentation fade because it promotes the degradation of melanosomes from the basal layer to the stratum corneum of the epidermis by inducing cathepsin K and cathepsin L2 expression.
Acknowledgment
We would like to thank Ms. Midori Watanabe (School of Bioscience and Biotechnology, Tokyo University of Technology) for her technical assistance in the experiments on the 3D model of the human epidermis.
References
- Quevedo WC, Holstein TJ (2006) General biology of mammalian pigmentation. In: The Pigmentary System: Physiology and Pathophysiology, 2nd ed, Blackwell Publishing, Oxford, UK, pp. 61-90.
- Prota G (1988) Progress in the chemistry of melanins and related metabolites. Med Res Rev 8(4): 525-556.
- Yatsu A, Ohbayashi N, Tamura K, Fukuda M (2013) Syntaxin-3 is required for melanosomal localization of Tyrp1 in melanocytes. J Invest Dermatol 133(9): 2237-2246.
- Hwang I, Park JH, Park HS, Choi KA, Seol KC, et al. (2013) Neural stem cells inhibit melanin production by activation of Wnt inhibitors. J Dermatol Sci 72(3): 274-283.
- Raposo G, Marks MS (2007) Melanosomes-dark organelles enlighten endosomal membrane transport. Nat Rev Mol Cell Biol 8(10): 786-797.
- Maeda K (2017) Large melanosome complex is increased in keratinocytes of solar lentigo. Cosmetics 4(4): 49.
- Sage J, Quéral DD (2013) Autophagy has a significant role in determining skin color by regulating melanosome degradation in keratinocytes. J Invest Dermatol 133(10): 2416-2424.
- Zeng H, Harashima A, Kato K, Gu L, Motomura Y (2017) Degradation of tyrosinase by melanosomal pH change and a new mechanism of whitening with propylparaben. Cosmetics 4(4): 43.
- Ebanks JP, Koshoffer A, Wickett RR, Schwemberger S, Babcock G, et al. (2011) Epidermal keratinocytes from light vs. dark skin exhibit differential degradation of melanosomes. J Invest Dermatol 131(6): 1226-1233.
- Chen N, Seiberg M, Lin CB (2006) Cathepsin L2 levels inversely correlate with skin color. J Invest Dermatol 126(10): 2345-2347.
- Maeda K, Fukuda M (1996) Arbutin: mechanism of its depigmenting action in human melanocyte cultures. J Pharmcol Exp Ther 276(2): 765-769.
- Ando H, Funasaka Y, Oka M, Ohashi A, Furumura M, et al. (1999) Possible involvement of proteolytic degradation of tyrosinase in the regulatory effect of fatty acids on melanogenesis. J Lipid Res 40(7): 1312-1316.
- Maeda K, Inoue N, Nishikawa H, Miki S, Shibuhara O, et al. (2003) Involvement of melanin monomers in the skin persistent UVA-pigmentation and effectiveness of Vitamin C ethyl on UVA-pigmentation. Journal of Japanese Cosmetic Science Society 27(4): 257-268.
- Maeda K, Tomita Y (2007) Mechanism of the inhibitory effect of tranexamic acid on melanogenesis in cultured human melanocytes in the presence of keratinocyte-conditioned medium. J Health Sci 53(4): 389-396.
- Maeda K (2008) Advances in development of skin whitening agents. Fragr J 36: 65-67.
- Gu L, Zeng H, Takahashi T, Maeda K (2017) In vitro methods for predicting chemical leukoderma caused by quasi-drug cosmetics. Cosmetics 4(3): 31.
- Özbek H (2005) The anti-inflammatory activity of the Foeniculum vulgare L. Essential oil and investigation of its median lethal dose in rats and mice. Int J Pharmacol 1(4): 329-331.
- Ahmed AF, Shi M, Lui C, Kang W (2019) Comparative analysis of antioxidant activities of essential oils and extracts of fennel (Foeniculum vulgare Mill.) seeds from Egypt and China. Food Sci Hum Wellness 8(1): 67-72.
- Abdesslem SB, Boulares M, Elbaz M, Moussa OB, St-Gelais A, et al. (2021) Chemical composition and biological activities of fennel (Foeniculum vulgare Mill.) essential oils and ethanolic extracts of conventional and organic seeds. J Food Process Preserv 45: e15034.
- Kalleli F, Rebey BI, Wannes AW, Boughaleb F, Hammami M, et al. (2019) Chemical composition and anti-oxidant potential of essential oil and methanol extract from Tunisian and French fennel (Foeniculum vulgare Mill.) seeds. J Food Biochem 43(8): e12935.
- Oktay M, Gülcin I, Küfrevioglu OI (2003) Determination of in vitro antioxidant activity of fennel (Foeniculum vulgare) seed extracts. LWT 36(2): 263-271.
- Hirahara F, Takai Y, Iwano H (1974) Antioxidative activity of various spices on oils and fats. Eiyogaku Zasshi 32(1): 1-8.
- Nakayama R, Nakatani N, Horiuchi H (1996) Antioxidative activity of constituents from fennel seeds. J Home Econ Jpn 47(12): 1193-1199.
- Tomita Y, S Shibahara S, Takeda A, Okinaga S, Matsunaga J, et al. (1991) The monoclonal antibodies TMH-1 and TMH-2 specifically bind to a protein encoded at the murine b-locus, not to the authentic tyrosinase encoded at the c-locus. J Invest Dermatol 96(4): 500-504.
- Morita T, Kato H, Iwanaga S, Takada K, Kimura T, et al. (1977) New fluorogenic substrates for α-thrombin, factor Xa, kallikreins, and urokinase. J Biochem 82(5): 1495-1498.
- Tahara Y, Date A, Makino T, Shimizu T, Yamaguchi M (2009) Cathepsin L activity analysis method for evaluation of skin conditions of human. Bunseki Kagaku 58(1): 15-19.
- Inubushi T, Kakegawa H, Kishino Y, Katunuma N (1994) Specific assay method for the activities of Cathepsin L-type cysteine proteinases. J Biochem 116(2): 282-284.
- Poole B, Ohkuma S (1981) Effect of weak bases on the intralysosomal pH in mouse peritoneal macrophages. J Cell Biol 90(3): 665-669.
- Glaumann H, Ahlberg J (1987) Comparison of different autophagic vacuoles with regard to ultrastructure, enzymatic composition, and degradation capacity–formation of crinosomes. Exp Mol Pathol 47(3): 346-362.
- Chen S, Dong H, Yang S, Guo H (2017) Cathepsins in digestive cancers. Oncotarget 8 (25): 41690-41700.






























