Experimental Study of the Effect of UVB Absorbers and Salicylic Acid Derivatives on the Release and Production of Interleukin-1α After UV Irradiation
Jiahao Huang1 and Kazuhisa Maeda1,2*
1Bionics Program, Tokyo University of Technology Graduate School, Japan
2School of Bioscience and Biotechnology, Tokyo University of Technology, Japan
Submission: August 14, 2021;Published: September 08, 2021
*Corresponding author: Kazuhisa Maeda, Bionics Program and School of Bioscience and Biotechnology, Tokyo University of Technology, Japan
How to cite this article: Jiahao H, Kazuhisa M. Experimental Study of the Effect of UVB Absorbers and Salicylic Acid Derivatives on the Release and Production of Interleukin-1α After UV Irradiation. JOJ Dermatol & Cosmet. 2021; 4(3): 555636. DOI: 10.19080/JOJDC.2021.04.555636
Abstract
The harmful effects of UVB radiation on the skin are approximately 1000 times higher than that of UVA radiation. UVB absorbers are used in sunscreen-based cosmetics to improve their safety and function. Ethylhexyl methoxycinnamate is the most widely used UVB absorber but has low photostability; moreover, it inhibits cell proliferation and DNA synthesis, which can accelerate mutagenic activity. Therefore, we explored UV absorbers that mimic the UV-absorption effect of ethylhexyl methoxycinnamate but without cell membrane-damaging and -inflammatory effects. IL-1α levels in the culture supernatant and IL-1α mRNA expression levels in a 3D human epidermis model were determined after applying UV absorbers before exposure to UV irradiation. Ethylhexyl methoxycinnamate increased IL-1α levels but not IL-1α mRNA expression in the culture medium after UV irradiation, indicating that this compound could damage the cell membrane. Both IL-1α levels in the cell culture medium and IL-1α mRNA expression were decreased with 2-carboxyphenyl salicylate and tranexamic acid salicylate treatments, regardless of UV irradiation. These results indicated that 2-carboxyphenyl salicylate and tranexamic acid salicylate inhibited IL-1α at the transcriptional level. Moreover, both these compounds exhibited anti-inflammatory effects in addition to UV absorption.
Keywords:UVB absorbers; UV spectrum; Cell proliferation; IL-1α; New UV absorber
Introduction
Recent studies show that the ozone layer is being depleted owing to an increase in vehicle pollutants and fluorocarbon emissions from air conditioners [1,2]. This depletion has led to the reduced ability of the ozone layer to absorb ultraviolet (UV) light. UV light has a wavelength of 290-400 nm and is divided into two categories based on the wavelength: UVB (290-320 nm) and UVA (320-400 nm), with UVB having the greatest effect on the skin [3]. Although the levels of UVA that reach the earth are greater than those of UVB, the effects of UVB on the skin are approximately 1000 times greater than that of UVA [4]. Additionally, UVB levels that reach the surface of the earth are continuously increasing. A part of the UVB radiation is reflected from the surface of the skin, but the remaining radiation passes through the stratum corneum and into skin cells, which can be hazardous to human health. The acute effects of UVB on the skin include DNA damage [5], cell damage from reactive oxygen species [6], and inflammation resulting from cytokine release [7] by cells in response to UVB exposure. These acute effects result in inflammation and erythema [8]. Acute skin swelling is also known as sunburn [9] and skin swelling is typically the result of inflammation or fluid buildup. Constant exposure to UVB results in dry and rough skin, visible and large wrinkles, freckle-like moles, and other photo-aging phenomena [10]. Much research has examined UV radiation and the development of skin cancer [11]. It has been reported that sunscreens reduce the incidence of cancer precursors, such as actinic keratosis and spinous cell carcinoma [12].
The development of skin malignancies is influenced by the amount and degree of UV radiation exposure beginning at a young age. UV exposure of skin is thought to be the cause of the development of several skin malignancies. Thus, it is important to avoid UV radiation in daily life from a young age. We reported that radiations with wavelengths in the 300-340-nm range cause hardening and reduced elasticity of collagen gels, with radiation at 330 nm exhibiting the most pronounced effect [13]. Therefore, sun protection, including direct exposure to sunlight by using an umbrella or wearing long sleeves together with the use of sunscreen-type cosmetics containing UV absorbers, must be considered to prevent skin damage by UV rays. The chemicals are used in the cosmetic industry are key ingredients in sun-protection formulations [14]. In this study, we focus on the efficacy and safety of UV absorbers. Since the 1980s, cases of skin injury caused by UV absorbers have been reported, and restrictions have been placed on the amount of UV absorbers used in various countries, including ethylhexyl methoxycinnamate, the most widely used UVB absorbent. In 1986, Itoh reported a case of contact dermatitis caused by ethylhexyl methoxycinnamate [15]. UV absorbers have been found to penetrate the stratum corneum and intercellular lipids into the skin [16-20], which raises concerns about skin damage. Although skin allergy caused by UV absorbers does not occur in all people, it is one of the problems of sunscreen cosmetics in daily use. A study of the systemic absorption and pharmacokinetics of sunscreen formulations containing six active ingredients [avobenzone, oxybenzone, octocrylene, homosalate, octisalate (ethylhexyl salicylate), and octinoxate (ethylhexyl methoxycinnamate)] applied to healthy subjects revealed that all six active ingredients were absorbed systemically. Moreover, plasma concentrations exceeded FDA thresholds that may exempt some additional safety studies of sunscreens [21].
When ethylhexyl methoxycinnamic acid absorbs UV light, its molecular structure isomerizes from the E-type to the Z-type, reducing its absorption capacity [22]. However, it can be absorbed rapidly through the skin and is detected in human urine, blood, and breast milk, which indicates that humans are systemically exposed to this compound [23,24]. Ethylhexyl methoxycinnamate showed weak estrogen receptor alpha agonism, but potent progesterone antagonism in cultures [25]. Ethylhexyl methoxycinnamate is an environmental endocrine disruptor that mimics estrogen and can disrupt thyroid function [24,26,27] and not a human health one [28]. The sale and distribution of sunscreens containing ethylhexyl methoxycinnamate and oxybenzone that harm coral reefs are banned in Hawaii [29], which can further affect its sale in other countries and regions. Therefore, finding an alternative UVB absorbent is imperative. Thus, the effects of 2-ethylhexyl salicylate and ethylhexyl triazone, which have the same UVB absorption wavelength range as ethylhexyl methoxycinnamate, were studied [30]. As ethylhexyl salicylate has a low UV absorption capacity, two salicylic acid derivatives, 2-carboxyphenyl salicylate and tranexamic acid salicylate, were analyzed in this study. We studied these two UV absorbers, which could mimic the UV-absorption effect of ethylhexyl methoxycinnamate, prevent cell-membrane damage, and exert anti-inflammatory effects. We report the design and evaluation of a novel UV absorber that exhibits both antiinflammatory and UV-absorption effects.
Materials and Methods
UV Absorbers
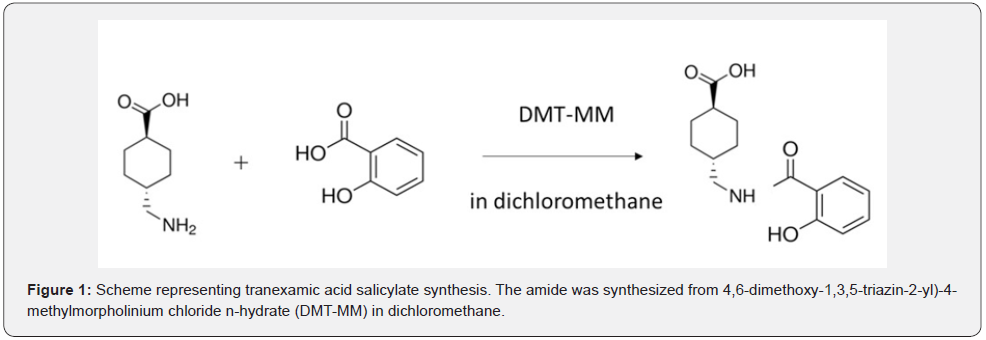
Ethylhexyl methoxycinnamate was purchased from FUJIFILM Wako Pure Chemical Corporation (Osaka, Japan). Ethylhexyl salicylate, ethylhexyl triazone, and 2-carboxyphenyl salicylate were purchased from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan). The synthesis scheme of tranexamic acid salicylate is shown in Figure 1. Tranexamic acid salicylate was synthesized using the following method: 0.005 mol (0.69 g) salicylic acid was dissolved in 100 mL methylene chloride and 0.005 mol (1.38 g) 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium chloride n-hydrate (FUJIFILM Wako Pure Chemical Corporation) was added. This mixture was stirred at room temperature for 1 d. Dichloromethane was distilled off using a rotary evaporator; 100 mL ethyl acetate and 100 mL H2O were added to the remaining residue. Once dissolved, the ethyl acetate layer was separated using a separating funnel. Ethyl acetate was distilled off using a rotary evaporator to obtain a residue. The target product was obtained by separation using a preparative column (Intersil® ODS-3, 20 x 50 mm, GL Sciences Inc., Tokyo, Japan). The molecular formula of the peak was determined by measuring the m/z of the target product using ESI-LC/MS (Instrumental Analysis Div., Global Facility Center, Creative Institution, Hokkaido University, Hokkaido, Japan).
Measuring UV-absorption Effects of the UV Absorbers
Ethylhexyl methoxycinnamate, ethylhexyl salicylate, 2-carboxyphenyl salicylate, and tranexamic acid salicylate were diluted with 50% ethanol [ethanol:H2O (1:1)] to 0.001 mol/L. Ethylhexyl triazone was diluted with olive oil to a concentration of 0.001 mol/L. The UV spectra were obtained by adding 100 μL of each solution into a UV-transmissive plate (Corning-Coster; Corning, NY, USA) and measuring the absorption from 290 to 390 nm using a POWERSCAN HT microplate reader (DS Pharma Biomedical Co. Ltd., Osaka, Japan).
Cell-growth Assay after UV Irradiation
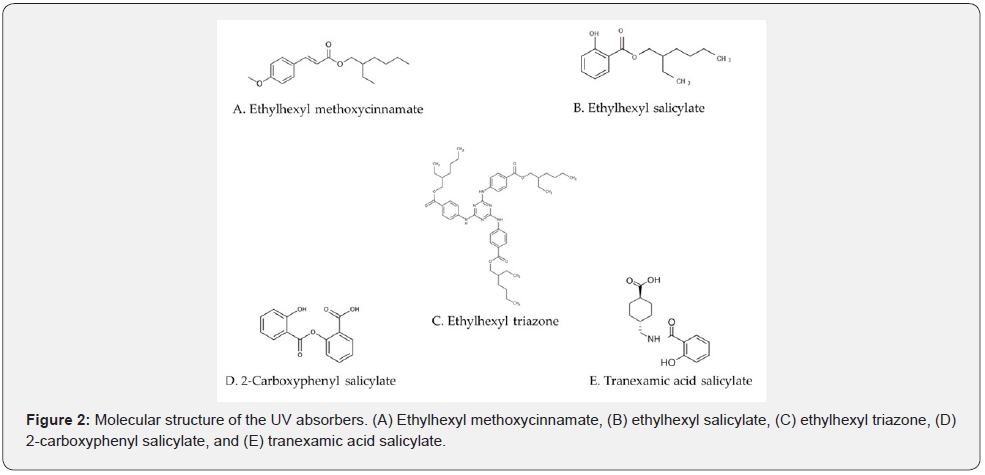
A human 3D epidermis model (Japan Tissue Engineering Co., Ltd., Aichi, Japan) cultured in 24-well plates was used, which was cultured in an incubator (5% CO2, 37°C) for 1 day. Either 100% dipropylene glycol (DPG) or 50% DPG [DPG:H2O (1:1)] was used as a solvent. Solutions (25 μL) of 0.1 mol/L (29 mg/mL) ethylhexyl methoxycinnamate (Figure 2A), 0.1 mol/L ethylhexyl salicylate (Figure 2B), 0.025 mol/L (20.6 mg/mL) ethylhexyl triazone (Figure 2C), and 0.1 mol/L (25.8 mg/mL) 2-carboxyphenyl salicylate (Figure 2D) were prepared in DPG; whereas 0.1 mol/L (27.3 mg/mL) tranexamic acid salicylate (Figure 2E) solution was prepared in 50% DPG and applied to the epidermis model. Either DPG or 50% DPG was used as a control group (n=3). One plate was irradiated using ultraviolet lamps (TL20S・BL/DMR BB-UVB wavelength: 280–380 nm, peak: 311-313 nm) from a height of 25 cm for 8 min. The other plate was not irradiated. The intensity of the irradiation at 305 nm was measured, and the exposure time was set so that the energy was 250 mJ/cm2. The plates were then cultured for 2 d in an incubator. Fifty microliters of Cell Counting Kit-8 solution (Dojindo Laboratories Co., Ltd., Kumamoto, Japan) was added to the wells and incubated for another 2 h before the absorption at 450 nm was measured using a microplate reader (POWERSCAN HT; DS Pharma Biomedical Co. Ltd., Osaka, Japan).
Measurement of Interleukin 1 Alpha (IL-1α) in Cell Culture Supernatants
Experimental conditions and steps, including cell cultivation, addition of UV absorbers, and the UV irradiation parameters were the same as those described in section 2.3. After incubation for two days, the amount of IL-1α in the cell culture supernatant was measured using an ELISA kit (Proteintech Group, Inc., Rosemont, IL, USA). Briefly, 100 μL of each standard and cell-culture supernatant was added to the wells of a 96-well plate and then sealed. The plate was incubated for 2 h at 37°C in a humidified environment. The cells were washed four times with 300 μL 1X wash buffer per well. Next, 100 μL of 1X detection antibody solution was added to each well. Plates were sealed and incubated for 1 h at 37°C in a humidified environment, after which 100 μL of 1X HRP-conjugated antibody was added to each well. The plate was sealed and incubated for a further 40 min at 37°C in a humidified environment. Then, 100 μL of 3,3´,5,5´-tetramethylbenzidine (TMB) substrate solution was added to each well and the plate was incubated for 15-20 min at 37°C in the dark. To stop the reaction, 100 μL of stop solution was added to each well in the same order as the TMB substrate and mixed by gently tapping the sides of the plate. Immediately after adding the stop solution, the absorbance of the solutions was determined using a microplate reader at 450 nm and 630 nm.
Measurement of IL-1α mRNA expression levels
After incubation for two days, total RNA was isolated from the 3D human epidermis model using an mRNA extraction kit (Qiagen K.K., Tokyo, Japan) according to the manufacturer’s instructions.
The expression levels of IL-1α were determined using a realtime reverse transcription PCR kit (Takara Bio, Otsu, Japan) on a QuantStudios® 5 real-time PCR system (Applied Biosystems, Foster City, CA, USA) according to the manufacturer’s protocol. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression was used as an endogenous control. Primers used to amplify IL1AS and GAPDH were purchased from Qiagen. Relative changes in mRNA expression levels were calculated using the 2−ΔΔCt method, and the levels were normalized to that of GAPDH. The experiment was performed in triplicate.
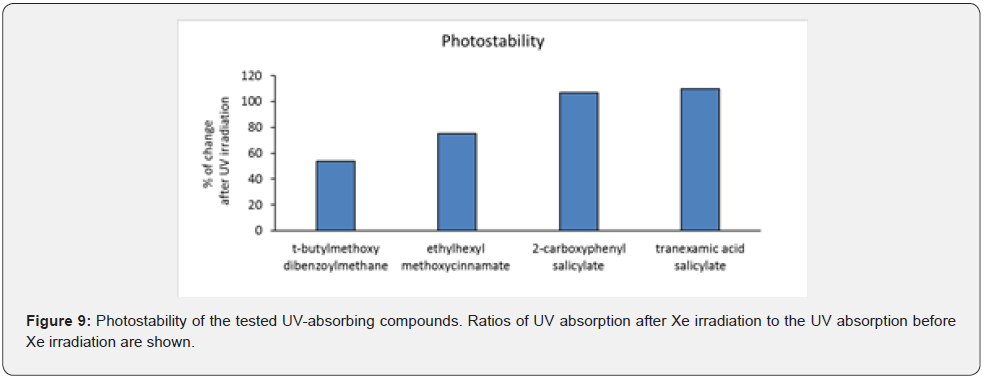
Photostability test
We dissolved 0.1 mg/mL t-butylmethoxydibenzoylmethane, ethylhexyl methoxycinnamate, 2-carboxyphenyl salicylate, and tranexamic acid salicylate in DPG. The t-butylmethoxydibenzoylmethane was used as a positive control. One hundred microliters of these solutions were added to the UV transmissive plate and the respective maximal absorptions at 360 nm, 310 nm, 310 nm, and 330 nm were measured using the microplate reader. After Xe irradiation for 50 min using a long-pass filter with 280 nm cutoff at a distance of 30 cm, which delivered approximately 25 times the amount of solar UV rays [13], the same respective absorptions were again measured using the microplate reader. Photostability was determined as the percentage change (%) after irradiation compared to before UV irradiation.
Data Analysis
IL-1α levels in the culture supernatant and IL-1α mRNA expression levels are expressed as mean ± standard deviation for the cell-growth assay. An unpaired t-test was performed to compare group means; P < 0.05 was considered statistically significant.
Result
Analysis of tranexamic acid salicylate
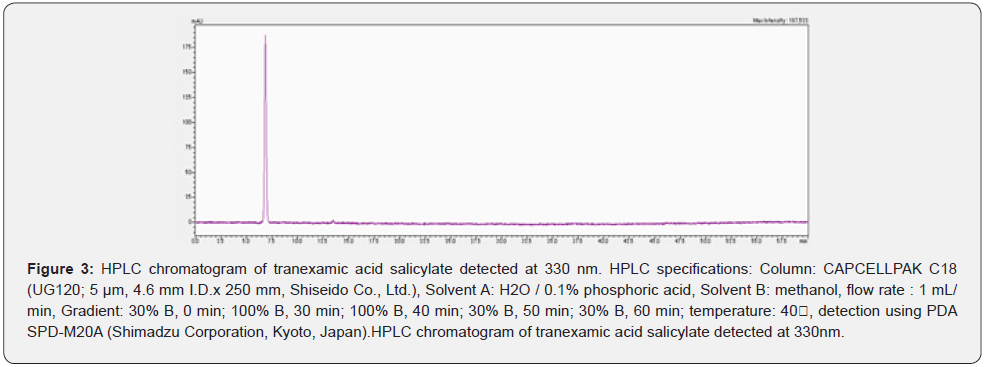
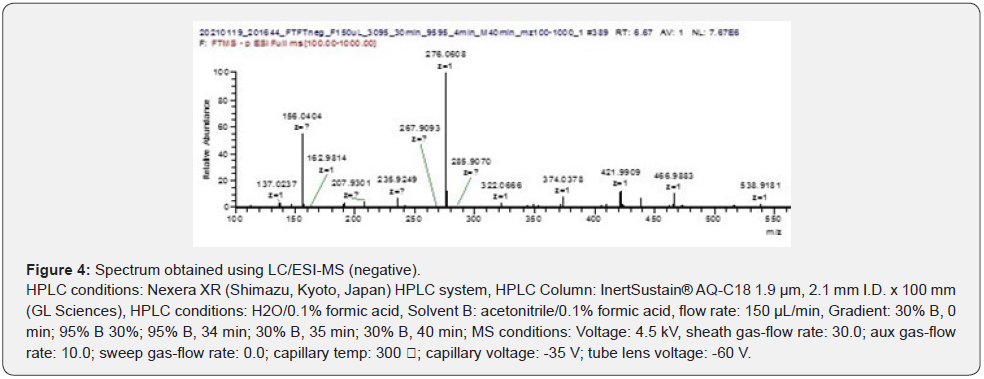
The sorted HPLC spectrum is shown in Figure 3. Figure 4 shows the LC / ESI-MS analysis results (negative) of the sorted peaks. The m/z value was measured as 276.0608. Tranexamic acid (C8H15NO2) 157.21 g/mol, salicylic acid (C7H6O3) 138.12 g/mol,and tranexamic acid salicylate (C15H19NO4) are calculated to be 277.314 g/mol and the product was determined to be tranexamic acid salicylate.
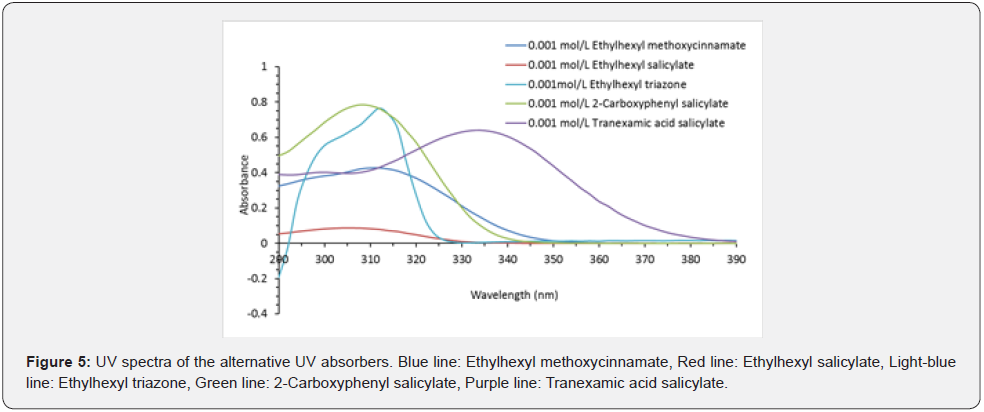
Different Ultraviolet Absorption Abilities of Alternative UV Absorbers
The results are shown in Figure 5. Ethylhexyl methoxycinnamate was used as a positive control, which had a maximum UV absorption of 0.43 at 311 nm in the UV wavelength range. Ethylhexyl salicylate had a maximum UV absorbance of 0.09 at 305 nm, whereas that of ethylhexyl triazone was 0.77 at 314 nm. In addition, 2-carboxyphenyl salicylate and tranexamic acid salicylate had maximum UV absorbances of 0.79 at 308 nm and 0.64 at 334 nm, respectively. The absorption of these compounds at 390 nm was negligible.
Cell Growth Assay After UV Irradiation
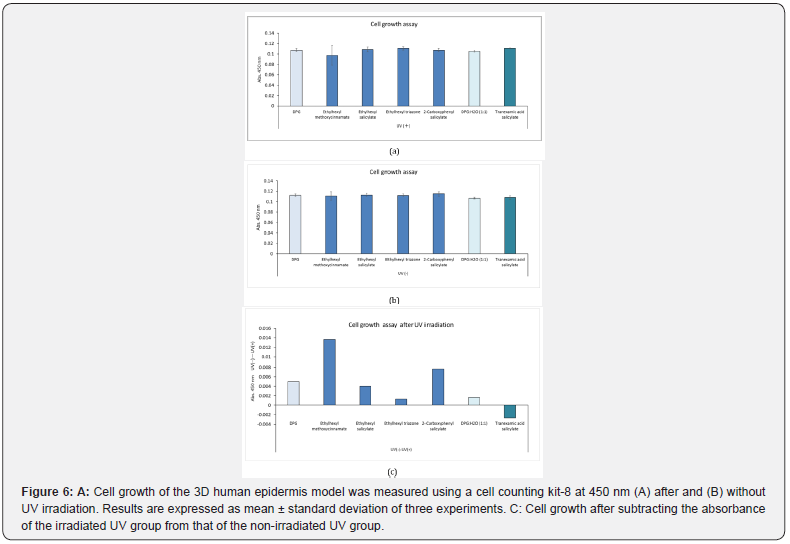
Each UV absorber was applied to the 3D human epidermis model, which was then irradiated with or without UV and cultured for 2 d before cells were stained for counting. The absorbances were determined at 450 nm (Figure 6A & 6B). In both the UVirradiated and unexposed groups, there was no significant difference in the absorbance value at 450 nm among the UV absorbers and their solvents (DPG or 50% DPG). The mean value of absorbance of the irradiated UV absorbers at 450 nm was subtracted from the mean value of non-irradiated UV absorbers at 450 nm. The highest value was reported in the group treated with ethylhexyl methoxycinnamate (Figure 6C). However, the number of cells was lower than that of DPG after irradiation with UV rays, but the difference was only 0.01.
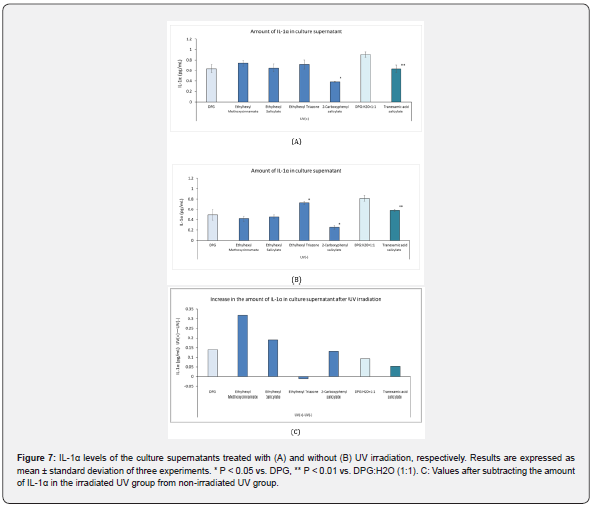
UV Irradiation-induced Cell Damage is Reduced with UV-Absorber Treatment
Figure 7A shows the amount of IL-1α in the culture supernatant of the 3D human epidermis model after the application of UV absorbers and subsequent exposure to UV irradiation. There was no significant difference in IL-1α levels in the supernatant among ethylhexyl methoxycinnamate, ethylhexyl salicylate, and ethylhexyl triazone, and their solvents (DPG or 50% DPG); however, 2-carboxyphenyl salicylate and tranexamic acid salicylate decreased IL-1α levels in the culture supernatant. Figure 7B shows the IL-1α levels in the culture supernatant of the 3D human epidermis model after the application of UV absorbers but without exposure to UV irradiation. There was no significant difference in the amount of IL-1α measured at 450 nm among all UV absorbers examined and their solvents (DPG or 50% DPG). Figure 7C shows the value after the amount of IL-1α in irradiated cultures treated with UV absorbers was subtracted from that in the non-irradiated cultures treated with UV absorbers. The amount of IL-1α in ethylhexyl methoxycinnamate increased after UV irradiation, while ethylhexyltriazone increased IL-1α release in cells unexposed to UV radiation but not after UV irradiation.
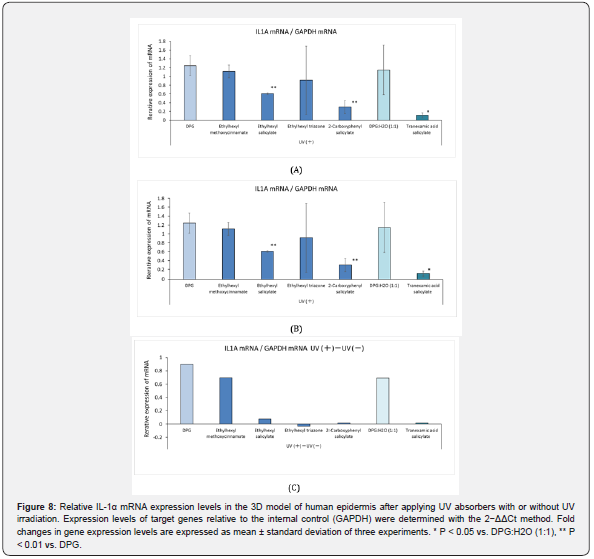
Relative IL-1α mRNA Expression in the 3D Human Epidermis Model After Application of the UV Absorbers
Figure 8 shows the relative levels of IL-1α mRNA expression in the 3D human epidermis model after application of the UV absorber with or without UV irradiation. In the UV-irradiation group, there was no significant difference in the relative IL-1α mRNA expression levels between ethylhexyl methoxycinnamate, ethylhexyl triazone, and DPG. However, ethylhexyl salicylate, 2-carboxyphenyl salicylate, and tranexamic acid salicylate suppressed IL-1α mRNA expression after UV exposure. In the unexposed group, there was no significant difference in the relative IL-1α levels among the UV absorbers and their solvents [DPG or DPG:H2O (1:1)].
Photostability of Alternative UV Absorbers
Figure 9 shows the photostability of t-butylmethoxydibenzoylmethane, ethylhexyl methoxycinnamate, 2-carboxyphenyl salicylate, and tranexamic acid salicylate. t-Butylmethoxydibenzoylmethane and ethylhexyl methoxycinnamate have poor photostability, whereas 2-carboxyphenyl salicylate and tranexamic acid salicylate have good photostability.
Discussion
The UV-absorption effect of 2-carboxyphenyl salicylate and tranexamic acid salicylate was similar to that of ethylhexyl methoxycinnamate and better than that of ethylhexyl salicylate. Tranexamic acid salicylate has an absorption wavelength from 300 nm to 360 nm, with a peak at 334 nm. Although radiation at a wavelength of 300–340 nm causes hardening and reduced elasticity of collagen gels, radiation at 330 nm had the most pronounced effect on the reduced elasticity of collagen gels [13]. Therefore, tranexamic acid salicylate is an effective compound for reducing photoaging of the dermis in addition to having an antiinflammatory effect. Results of the cell-growth assay suggested that ethylhexyl methoxycinnamate did not inhibit cell growth after UV radiation. Moreover, ethylhexyl salicylate, ethylhexyl triazone, 2-carboxyphenyl salicylate, and tranexamic acid salicylate did not inhibit cell growth after UV radiation. Ethylhexyl methoxycinnamate inhibits cell growth and DNA synthesis in cultured human cells and retards cell-cycle progression at the G1 phase when added to cultured cells at doses of 25-100 pg/mL [31]. This concentration could be achieved in vivo when sunscreens containing ethylhexyl methoxycinnamate were used on the skin [31] and were degraded by sunlight (photodegradation), thereby producing toxic byproducts [32].
Membrane damage is a major factor involved in the release of extracellular IL-1α, because it does not have the signal sequence of normal secreted proteins. Therefore, it is considered to be indicative of inflammation-induced cell damage. By comparing IL- 1α levels in the cell-culture supernatants collected from the UVirradiated and non-irradiated groups, we found that ethylhexyl methoxycinnamate application followed by UV irradiation increased IL-1α levels in the cell-culture medium without an increase in the mRNA expression. This finding indicated that ethylhexyl methoxycinnamate could damage cell membranes when exposed to UV irradiation. Both IL-1α levels in the cell-culture medium and IL-1α mRNA expression decreased significantly after the application of 2-carboxyphenyl salicylate and tranexamic acid salicylate, irrespective of UV irradiation. These results indicated that 2-carboxyphenyl salicylate and tranexamic acid salicylate inhibited IL-1α at the transcription level. Moreover, these findings were suggestive of the anti-inflammatory effect of 2-carboxyphenyl salicylate and tranexamic acid salicylate, which are consistent with the reported use of 2-carboxyphenyl salicylate in the treatment of inflammatory conditions, such as rheumatoid arthritis and osteoarthritis, among others [33].
Salicylates inhibit IκB kinase, thereby inhibiting the nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB) cascade and decreasing the production of inflammatory cytokines (IL- 6, TNF-α, and C-reactive protein) and insulin resistance [34]. A similar mechanism can be proposed for the inhibition of IL-1α transcription by these UV absorbers. In addition, IL-1α levels in the cell-culture medium increased in cultures in which ethylhexyl triazone was applied without exposure to UV irradiation; however, an increase was not observed upon exposure to UV irradiation. These results indicated that ethylhexyl triazone does not damage the cell membrane; however, IL-1α mRNA expression levels increased in the non-irradiated 3D human epidermis model, which suggested a possibility of inflammation after the application of ethylhexyl triazone. Although other studies indicate that ethylhexyl triazone suppresses inflammation after UV irradiation [35], there are no reports on whether or not this compound can induce inflammation without UV exposure; therefore, it is necessary to investigate this effect in future studies. These results were obtained in vitro using the 3D human epidermis models. Further in vivo studies in humans are needed.
Conclusion
Our results showed that 2-carboxyphenyl salicylate had an anti-inflammatory potential and that its UV absorption effects were similar to those of ethylhexyl methoxycinnamate and ethylhexyl triazone. Tranexamic acid salicylate is also effective against photoaging of the dermis in addition to its shielding and anti-inflammatory effects. Additional studies are required to determine the esterification ability of tranexamic acid salicylate to meet the oil-solubility criterion for UV absorbers.
References
- McKenzie RL, Aucamp PJ, Bais AF, Björn LO, Ilyas M, et al. (2011) Ozone depletion and climate change: impacts on UV radiation. Photochem Photobiol Sci 10(2): 182-198.
- Bais AF, McKenzie RL, Bernhard G, Aucamp PJ, Ilyas M, et al. (2015) Ozone depletion and climate change: impacts on UV radiation. Photochem Photobiol Sci 14(1): 19-52.
- Herzog B, Hüglin D, Borsos E, Stehlin A, Luther H (2004) New UV absorbers for cosmetic sunscreens – A breakthrough for the photoprotection of human skin. CHIMIA International Journal for Chemistry 58(7-8): 554-559.
- Pearse AD, Gaskell SA, Marks R (1987) Epidermal changes in human skin following irradiation with either UVB or UVA. J Invest Dermatol 88(1): 83-87.
- Orazio J, Jarrett S, Amaro OA, Scott T (2013) UV radiation and the skin. Int J Mol Sci 14(6): 12222-12248.
- Pfeifer GP, Besaratinia A (2012) UV wavelength-dependent DNA damage and human non-melanoma and melanoma skin cancer. Photochem Photobiol Sci 11(1): 90-97.
- Suh DH, Kwon TE, Youn J I (2002) Changes of comedonal cytokines and sebum secretion after UV irradiation in acne patients. Eur J of Dermatol 12(2): 139-144.
- Ryckaert S, Roelandts R (1998) Solar Urticaria- A report of 25 cases and difficulties in phototesting. Arch Dermatol 134(1): 71-74.
- Whiteman D, Green A (1994) Melanoma and sunburn. Cancer Causes Control 5(6): 564-572.
- Kobayashi S (2006) UVB-induced skin damage and the protection/treatment-Effects of a novel, hydrophilic γ-tocopherol derivative. Yakugaku Zasshi 126(9): 677-693.
- Ratushny V, Gober MD, Hick R, Ridky TW, Seykora JT (2012) From keratinocyte to cancer: the pathogenesis and modeling of cutaneous squamous cell carcinoma. J Clin Invest 122(2): 464-472.
- Farmer KC, Naylor MF (1996) Sun exposure, sunscreens, and skin cancer prevention: a year-round concern. Ann Pharmacother 30(6): 662-673.
- Maeda K (2018) Analysis of ultraviolet radiation wavelengths causing hardening and reduced elasticity of collagen gels in vitro. Cosmetics 5(1): 14.
- Berkey C, Oguchi N, Miyazawa K, Dauskardt R (2019) Role of sunscreen formulation and photostability to protect the biomechanical barrier function of skin. Biochem Biophys Rep 19: 100657.
- Itoh M, (1997) A case of photocontact dermatitis due to Parsol MCX. Environ Dermatol 4(2): 146-150.
- Hayden CGJ, Cross SE, Anderson C, Saunders NA, Roberts MS (2005) Sunscreen penetration of human skin and related keratinocyte toxicity after topical application. Skin Pharmacol Physiol 18(4): 170-174.
- Treffel P, Gabard B (1996) Skin penetration and sun protection factor of ultra-violet filters from two vehicles. Pharm Res 13(5): 770-774.
- Tippavajhala VK, de Oliveira Mendes T, Martin AA (2018) In vivo human skin penetration study of sunscreens by confocal Raman spectroscopy. AAPS PharmSciTech 19(2): 753-776.
- Benson HAE, Mohammed YH, Walters KA, Roberts MS (2021) Chapter 31 Percutaneous Absorption of Sunscreens, In: Dragićević N, Maibach H (Eds.), Percutaneous Absorption Drugs, Cosmetics, Mechanisms, Methods. CRC Press, BocaRaton London New York, USA, pp.417-434.
- Vasu P, Maibach H (2021) Chapter 32 Sunscreen Percutaneous Penetration in Vivo in Man, In: Dragićević N, Maibach H (Eds.), Percutaneous Absorption Drugs, Cosmetics, Mechanisms, Methods. CRC Press, BocaRaton London New York, pp.435-446.
- Matta MK, Florian J, Zusterzeel R, Pilli NR, Patel V, et al. (2020) Effect of sunscreen application on plasma concentration of sunscreen active ingredients: A randomized clinical trial. JAMA 323(3):256-267.
- Gonzalez H, Wahlberg NT, Strömdahl B, Juzeniene A; Moan J, et al. (2007) Photostability of commercial sunscreens upon sun exposure and irradiation by ultraviolet lamps. BMC Dermatol 7: 1.
- Axelstad M, Boberg J, Hougaard KS, Christiansen S, Jacobsen PR, et al. (2011) Effects of pre-and postnatal exposure to the UV-filter Octyl Methoxycinnamate (OMC) on the reproductive, auditory and neurological development of rat offspring. Toxicol Appl Pharmacol 250(3): 278-290.
- Schlumpf M, Schmid P, Durrer S, Conscience M, Maerkel K, et al. (2004) Endocrine activity and developmental toxicity of cosmetic UV filters-an update. Toxicology 205(1-2): 113-122.
- Schreurs RHMM, Sonneveld E, Jansen JHJ, Seinen W, Burg BVD (2005) Interaction of polycyclic musks and UV Filters with the estrogen receptor (ER), androgen receptor (AR), and progesterone receptor (PR) in reporter gene bioassays. Toxicol Sci 83(2): 264-272.
- Schlumpf M, Cotton B, Conscience M, Haller V, Steinmann B, et al. (2001) In vitro and in vivo estrogenicity of UV screens. Environ Health Perspect 109(3): 239-244.
- Krause M, Klit A, Blomberg JM, Søeborg T, Frederiksen H, et al. (2012) Sunscreens: are they beneficial for health? An overview of endocrine disrupting properties of UV-filters. Int J Androl 35(3): 424-436.
- Call for data on ingredients with potential endocrine-disrupting properties used in cosmetic products. Internal Market, Industry, Entrepreneurship and SMEs. European Cpmmision.
- Siller A, Blaszark SC, Lazar M (2018) Update about the effects of the sunscreen ingredients oxybenzone and octinoxate on humans and the environment. Plast Surg Nurs 38(4): 158-161.
- Kuroda A, Sakai K, Yahagi S, Mukawa T, Sato N, et al. (2019) Surface structures of cosmetic standard poly methyl methacrylate UV evaluation plates and their influence on the in vitro evaluation of UV protection abilities of cosmetic sunscreens. J Oleo Sci 68(2): 175-182.
- Xu C, Parsons P (1999) Cell cycle delay, mitochondrial stress and uptake of hydrophobic cations induced by sunscreens in cultured human cells. Photochem Photobiol 69(5): 611-616.
- Stein HV, Berg CJ, Maung JN, Connor LE, Pagano AE, et al. (2017) Photolysis and cellular toxicities of the organic ultraviolet filter chemical octyl methoxycinnamate and its photoproducts. Environ Sci. Process Impacts 6.
- Anderson K, Wherle L, Park M, Nelson K, Nguyen L (2014) Salsalate, an old, inexpensive drug with potential new indications: a review of the evidence from 3 recent studies. American Health & Drug Benefits 7(4): 231-235.
- Hayden MS, Ghosh S (2012) NF-κB, the first quarter-century: remarkable progress and outstanding questions. Genes Dev 26(3): 203-234.
- Couteau C, Chauvet C, Paparis E, Coiffard L (2012) UV filters, ingredients with a recognized anti-inflammatory effect. PLoS ONE 7(12): e46187.






























