Efficacy and Safety of Autologous Platelet Concentrate in the Treatment of Photoaging on the Back of the Hands
Israel Alfonso Trujillo*, Yetter Cruz Leon, Charllonne Angelica Marte Arias, Yaquelin Luciana Morales Novo, Jesus Lazaro Diego De la Campa, Angela Rosa Gutierrez Rojas, Julio Antonio López Silva and Daymi Serpa Almaguer
Clinical Surgical Hospital Hermanos Ameijeiras, Cuba
Submission: April 08, 2021;Published: April 26, 2020
*Corresponding author: Israel Alfonso Trujillo, Clinical Surgical Hospital Hermanos Ameijeiras. Havana, Cuba
How to cite this article: Israel A T, Yetter C L, Charllonne A M A, Yaquelin L M N, Jesus L D D l C, et al. Efficacy and Safety of Autologous Platelet Concentrate in the Treatment of Photoaging on the Back of the Hands. JOJ Dermatol & Cosmet. 2021; 4(1): 555628. DOI: 10.19080/JOJDC.2020.04.555628
Abstract
Introduction: From the fourth decade of life (especially on the back of the hands) the skin becomes dry and brittle, spots appear, the venous network increases and soft tissues decrease.
Objectives: To evaluate the efficacy and safety of intradermal microinjection of autologous platelet concentrate (APC) in the treatment of rejuvenation of the back of the hands.
Method: An observational, analytical and longitudinal study was carried out in 60 patients from the Hospital Clínico Quirúrgico: “Hermanos Ameijeiras”, in the period between March 1, 2017 and March 31, 2020. The treatment was applied monthly for 1 year. The final evaluation was carried out 3 months after the end of the treatment.
Results: 51 women with an average age of 45 ± 4.3 years were treated. After treatment, there were significant changes in the Glogau photodamage scale (p = 0.012), in the global scale of aesthetic improvement (p <0.001) and in the scale of the degree of involvement of the volume of the hand (p = 0.018). The adverse events found were pain, inflammation and ecchymosis. The degree of satisfaction reported by the patients was good (26.6%) and very good (73.4%) (p <0.001).
Conclusion:The autologous platelet concentrate proved to be effective and safe in reducing the signs of cutaneous aging on the back of the hands, associated with a high degree of patient satisfaction.
Keywords:Platelet-rich plasma; Rejuvenation; Photoaging; Autologous platelet concentrate
Introduction
From the fourth decade of life, the skin becomes dry and brittle, spots appear, red vein increases and soft tissues decrease, especially on the back of the hands. To achieve rejuvenation of the hands, the aesthetic doctor must combine and adapt various treatments. Platelet-rich plasma (PRP) and its growth factors (GF) have a recognized revitalizing, regenerative and biorepairing effect, [1,2] however few studies objectively evaluate their efficacy, which led to the realization of the present investigation.
Goals
The primary objective was: to determine the effectiveness and safety of the microinjection of autologous platelet concentrate (APC) in the treatment of photoaging of the back of the hands and the secondary objectives were: 1) to evaluate the clinical response to treatment, 2) to evaluate type and intensity of adverse events that occur and 3) describe the degree of patient satisfaction.
Method
An observational, analytical, longitudinal study was carried out in 60 patients at the Hospital Clínico Quirúrgico: “Hermanos Ameijeiras”, in the period between March 1, 2017 and March 31, 2020. Treatment with APC was applied monthly for 12 months. Three months after the end of the treatment, the response to it was sent (final evaluation), comparing the current state (soft tissues, veins, tendons and skin) with the initial state; for this, the patient had to attend the scheduled consultation. Throughout the study there was a rigorous control of adverse reactions. Before and after proceeding, the platelets were quantified to know the quality of the applied product (the average concentration of the platelets after advancing 10.8 times its initial value). Microbiological culture of the extracted plasma was performed to guarantee that a sterile germ product was administered.
Inclusion criteria
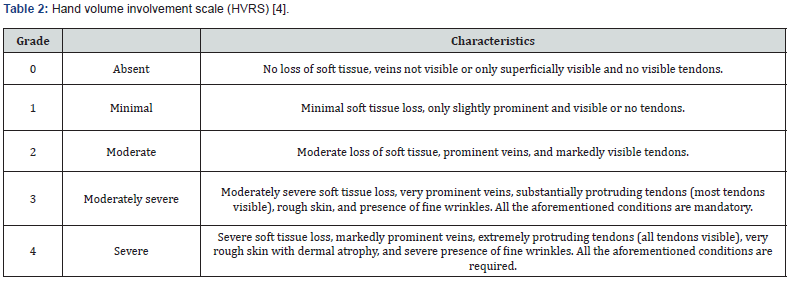
Patients between 20 and 60 years old, of any sex and skin phototype, skin photoaging grade II, III and IV (Glogau classification) (Table 1), [3] grade 1 to 3 according to scale of the degree of affectation of the volume of the (HVRS) (Table 2), [4] normal complementary tests (hemogram with differential, coagulogram, blood chemistry and serology for HIV, hepatitis B and C), with signed informed consent.
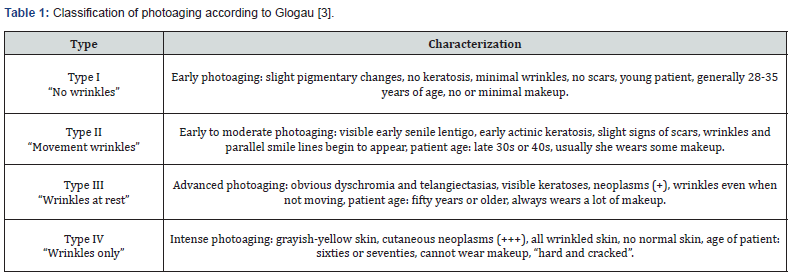
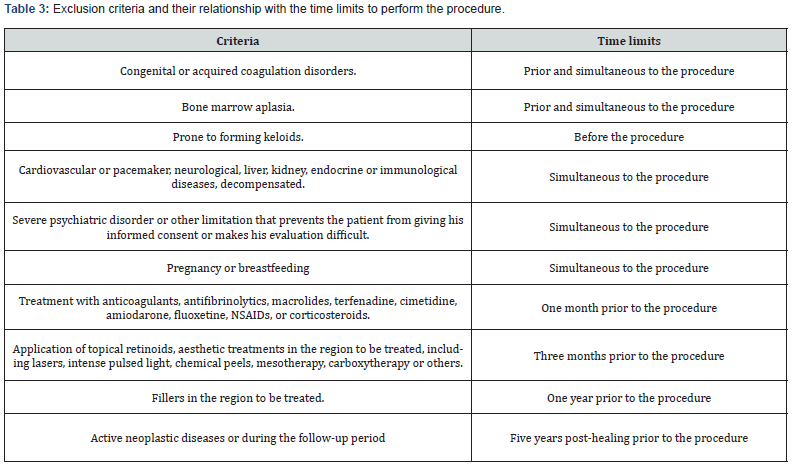
Exclusion criteria (Table 3)
Elimination criteria
Patients who wish to abandon the study, presence of an adverse event and / or complication that prevents continuing with the treatment or patients who have missed a treatment session.
Procedures
Once the patients gave informed consent, the included subjects registry template and the investigator’s internal registry were filled out. All information on the included patients was compiled in the data collection notebook. The blood was extracted (500 milliliters), then the APC was obtained with the Rotixa centrifuge (221 mm radius) according to international standards [5]. To obtain the APC, a first light centrifugation of the whole blood was carried out in the plastic bag for 3 minutes at 2800 rpm at 22 oC, with a centrifugation force of 2000 g, in this way 250 ml of red blood cells and 250 ml were obtained of PRP; then a second weighted centrifugation was performed on the PRP in the plastic bag for 5 minutes at 4500 rpm at 22 oC, with a centrifugation force of 5000 g. Once the heavy centrifugation had been carried out, the supernatant plasma was transferred through the tubes that have the plastic bags for blood collection and only 10 ml were left and it is in said volume that by shaking the platelets that were deposited in the cell were resuspended. bottom of the bag as results of the centrifugation procedure. Subsequently, the red blood cells were returned to the patients and finally a microinjection of 10 milliliters of the APC was performed, distributed between the back of the hands, the entire facial area, V of the décolleté and neck.
Asepsis and antisepsis of the back of the hands were performed. Radial and ulnar nerve block was performed by subcutaneous infiltration of 3 ml of 2% lidocaine close to the styloid process of the radius (anatomical tobacco box) and another 3 ml of 2% lidocaine at the level of the styloid process of the ulna (between the flexor carpi ulnaris tendon and the ulnar artery). Once the APC was activated with 10% calcium gluconate (concentration 10:1), it was administered by combining cannulas and needles. Punctures were made with a 20G needle at the level of the dorsal surface of the second, third and fourth interdigital spaces and through these punctures a 1 mm × 10 cm long cannula with a blunt tip was introduced, with which the APC was administered by subcutaneously (fan and backtracking techniques). Subsequently, with a 25G × 16 mm hypodermic needle and 1 ml syringes, intradermal injections of approximately 1.5 ml were administered at a distance of 1.5 to 2 mm between each application area (point-topoint, fan, backtrace and nappage).
Variables related to the response to treatment
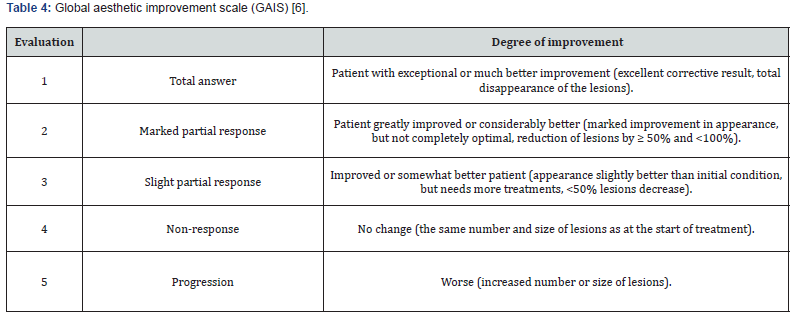
The response to treatment was evaluated taking into account the clinical examination of the patient, using the Glogau photodamage scale (Table 1),[3] the global aesthetic improvement scale (GAIS) (Table 4)[6] and the scale of the degree of involvement of the volume of the hand (Table 2)[4].
Adverse events
Adverse events reported in the reviewed literature are pain, edema, and ecchymosis at the microinjection site [1,2].
Classification of adverse events (Table 5)[7]
Degree of satisfaction of patients to treatment
The degree of satisfaction (PSSS) of the patients with the treatment was evaluated taking into account what was reported by the patient according to the scale (Table 6) [8].
Bioethical considerations
The protocol was submitted to the consideration and approval of a Review and Ethics Committee for Clinical Research created for this purpose, which evaluated it from an ethical point of view. Additionally, this protocol was submitted to scientific and methodological review and approval by the Institutional Scientific Council of the Hospital Clínico Quirúrgico “Hermanos Ameijeiras”.
Statistical methods used
The medical records of the patients included in the study were stored in the Department’s file. With the information gathered, a Microsoft Office version XP database in Excel format was created, which was exported to the SPSS version 21.0 system for analysis. To summarize the information of the study sample, the arithmetic mean, standard deviation and minimum and maximum values will be used. The Student’s t test was used for all quantitative variables. For all qualitative variables (degree of aesthetic improvement, degree of affectation of the volume of the hand and degree of satisfaction), the absolute numbers and percentages were calculated before and after the treatment, which were compared using Pearson’s Chi-square test. In all the hypothesis tests carried out, a significance level α = 0.05 was performed.
Sample’s size calculation
The sample size was calculated using the C4-Study Design Pack computerized program. (C4- SDP) for sample size calculation (CTM). Version 1.1 ® Glaxo Wellcome. SA; [9] considering the following values: percentage of success reported in the literature 70%, percentage of success in the current study of 80%. With an alpha error of 0.05, a power of 80% and covering a loss of 5% of the patients, it was necessary to have 60 subjects in total.
Results
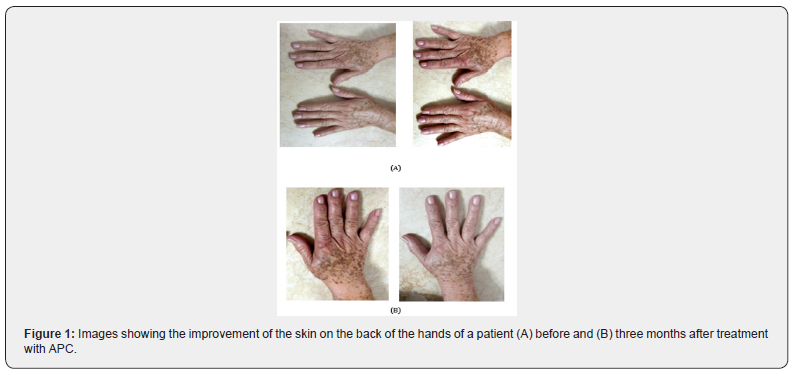
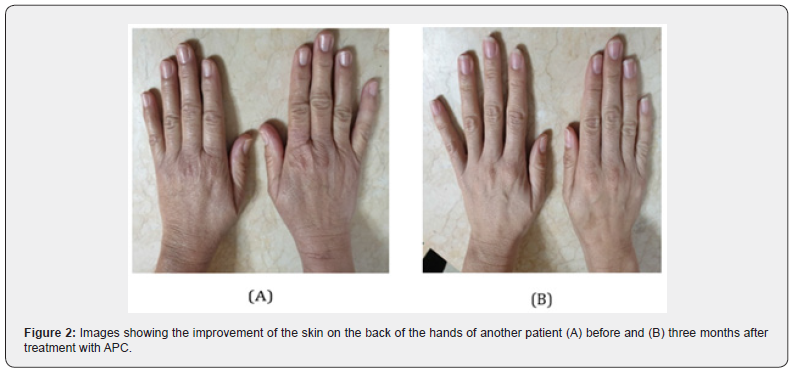
The study sample consisted of 60 women with skin phototypes between II and IV. The average age ranged around 45 ± 4.3 years (Table 7). Regarding the Glogau Photodamage Scale, 51 patients were classified as grade III, and 9 as grade II before the start of the study. After treatment, 33/51 (64.7%) patients who were classified as grade III were reclassified as grade II and 6/9 (66.6%) patients who were classified as grade II were reclassified as grade I (p = 0.012); the rest of the patients remained in the same grade assigned before treatment. According to the Global Esthetic Improvement Scale, there were significant changes after treatment (p <0.001); 3/60 (5%) patients achieved a total response, 37/60 (61.6%) patients achieved a marked partial response, and 20/60 (33.3%) patients achieved a slight partial response (Figure 1& 2). Regarding the Scale of the degree of involvement of the volume of the hand, 51 patients were classified as grade III, 6 as grade II and 3 as grade I before the start of the study.
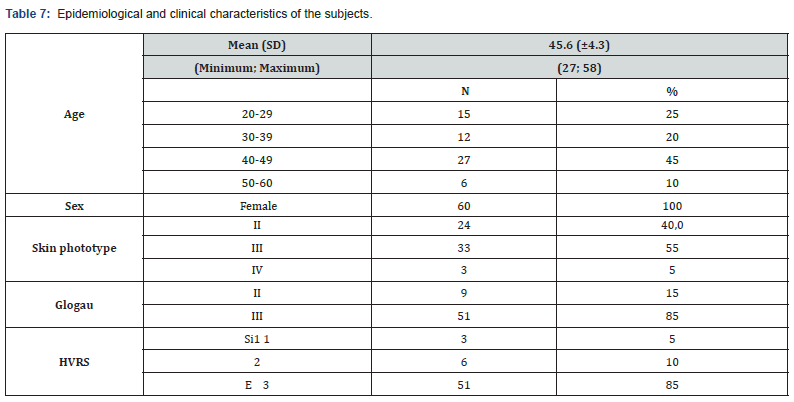
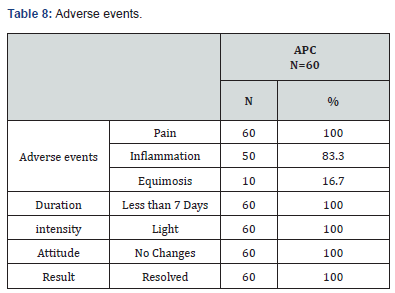
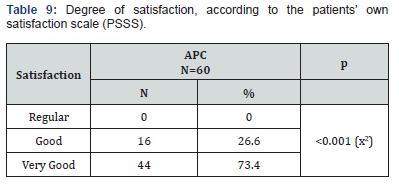
After treatment, 35/51 (68.6%) patients who were classified as grade III were reclassified as grade II, 4/6 (66.6%) patients who were classified as grade II were reclassified as grade I and 2 / 3 (66.6%) patients who were classified as grade I were reclassified as grade 0 (p = 0.018); the rest of the patients remained in the same grade assigned before treatment. All the patients reported some adverse event (pain, inflammation and ecchymosis), which were of slight intensity, did not imply changes before the intervention and were completely resolved. The pain occurred during the procedure and disappeared immediately after the completion of the procedure (100%), the inflammation (83.3%) lasted 2 to 3 days and the ecchymosis at the puncture sites (16.7%) were infrequent and of short duration (five to seven days in duration) (Table 8). Of the 60 patients treated with APC, 16/60 patients (26.6%) reported a good degree of satisfaction and a very good degree of satisfaction 44/60 patients (73.4%), because they achieved evident improvement with respect to their condition initial (Table 9).
Discussion
Platelets play a vital role in initiating hemostasis and wound healing. In response to tissue and vascular damage, a platelet plug is formed, with the subsequent release from its alpha granules of more than 30 biologically active proteins, including: transforming growth factor β, platelet-derived factor, growth factor, vascular endothelial growth factor, fibroblast growth factor, and epithelial cell growth factor. These factors not only aid in clotting but also drive angiogenesis and promote tissue repair and regeneration [10]. Multiple authors have shown that topical application or intradermal injection of PRP and its growth factors produce favorable cutaneous changes: clinical (restores its vitality, increases its thickness, recovers its elastic consistency, improves blood circulation, increases its smoothness, decreases its wrinkles and improves their appearance) [2,10,11] histopathological (increases the number of fibroblasts, collagen fibers and blood vessel basement membranes) [2,10,12] immunohistochemical (improves collagen expression type I, III and IV) [13-15] and the genetic material (through the polymerase chain reaction expression of three target genes, such as: collagen IA, matrix metalloproteinase gene 1 and protein rich in proline of keratinocytes) [13,14]. PRP has been used as an adjunct to multiple skin rejuvenation treatments (lipotransference, laser, peeling, lifting).
In all the studies reviewed these treatments expected a higher response when combined than alone [12,16-18], PRP has also been used to reduce the intensity and duration of adverse events in these modalities. In a study by Kim H. and Gallo J, they were able to objectively reduce erythema and edema after treatment with fractional carbon dioxide laser by administering intradermal PRP (P = 0.02), associated with fewer subjective symptoms reported by patients (itching, burning and pain) [18]. There is only one previous report on the use of PRP as a rejuvenating monotherapy of the back of the hands, which was carried out by Cabrera-Ramírez JO and collaborators who applied 3 sessions of subcutaneous PRP with a cannula on the back of the hands. At the end of the treatment, the subjects had clinical improvement in the Fitzpatrick wrinkle and elastosis classification scale (p <0.001) and in the Glogau photodamage scale (p = 0.01) and a histological increase in the number of fibroblasts (p = 0.000), number of vessels (p = 0.000) and amount of collagen (p = 0.27) [2]. In our study there was clinical improvement in the Glogau photodamage scale (p = 0.012), in the global scale of aesthetic improvement (p = 0.001) and in the scale of the degree of involvement of the volume of the hand (p = 0.018).
Conclusion
The application of autologous platelet concentrate proved to be effective and safe in reducing the signs of skin aging on the back of the hands, associated with a high degree of patient satisfaction.
References
- Fernández CA, Alfageme RF, Burón ÁI, Rodríguez SR, Villegas FC (2013) Bioestimulación cutánea con plasma rico en plaquetas autólogo. Estudio controlado con ecografía. Piel 28(2): 69-74.
- Cabrera RJO, Puebla MAG, González OA, García MD, Cortés JA, et al. (2017) Plasma rico en plaquetas en el tratamiento del fotodaño cutáneo en las manos. Actas Dermosifiliogr 108(8): 746-751.
- Glogau RG (1996) Aesthetic and anatomic analysis of the aging skin. Semin Cutan Med Surg 15(3): 134-138.
- Hun LJ, Su CY, Soo PE, Seo KJ, Seok KM, et al. (2019) A novel photonumeric hand grading scale for hand rejuvenation. Arch Plast Surg 46(4): 359-364.
- Nester T, AuBuchon JP (2013) Decisiones en hemoterpia y sus resultados. En: American Association of Banks Blood AABB. Manual Técnico de la AABB. 17a Ed. Buenos Aires: Asoc. Argentina Hemoterapia e Inmunohemat.
- Savoia A, Accardo C, Vannini F, Pascale B, Baldi A (2014) Outcomes in thread lift for facial rejuvenation: a study performed with happy lift revitalizing. Dermatol Ther (Heidelb) 4(1): 103-114.
- Moher D, Hopewell S, Schulz KF, Montori V, Gotzsche PC, et al. (2010) CONSORT 2010 Explanation and Elaboration: updated guidelines for reporting parallel group randomised trials. BMJ 340: c869.
- Larson L, Rovers J, Mackeigan L (2002) Patient satisfaction with pharmaceutical care: update of a validated instrument. J Am Pharm Assoc 42(1): 44-50.
- C4-Study Design Pack (C4- SDP) para el cálculo de tamaño de muestra (CTM). Versión 1.1®. Desarrollado por: Departamento de Biometría de Glaxo Wellcome.
- Chamata ES, Bartlett EL, Weir D, Rohrich RJ (2021) Platelet-Rich Plasma: Evolving Role in Plastic Surgery. Plast Reconstr Surg 147(1): 219-30.
- Devereaux J, Dargahi N, Fraser S, Nurgali K, Kiatos D (2020) Leucocyte-Rich Platelet-Rich Plasma Enhances Fibroblast and Extracellular Matrix Activity: Implications in Wound Healing. Int J Mol Sci 21(18): 6519.
- El‐Domyati M, Abdel‐Wahab H. Hossam A (2018) Microneedling combined with platelet‐rich plasma or trichloroacetic acid peeling for management of acne scarring: A split‐face clinical and histologic comparison. J Cosmet Dermatol 17(1): 73-83.
- Draelos ZD, Rheins LA, Wootten S, Kellar RS, Diller R (2019) Pilot study: Autologous platelet‐rich plasma used in a topical cream for facial rejuvenation. J Cosmet Dermatol 18(5): 1348-1352.
- Souza MV, Silva MB, Pinto O, Lima MB, Crepaldi J, et al. (2015) Immunohistochemical Expression of Collagens in the Skin of Horses Treated with Leukocyte-Poor Platelet-Rich Plasma. Biomed Res Int 2015: 893485.
- Arora NS, Ramanayake T, Ren YF, Romanos GE (2009) Platelet-rich plasma: a literatura review. Implant Dent 18(4): 303-310.
- Modarressi A (2013) Platlet Rich Plasma (PRP) Improves Fat Grafting Outcomes. World J Plast Surg 2(1): 6-13.
- Puri N (2015) Platelet rich plasma in dermatology and aesthetic medicine. Our Dermatol Online 6(2): 207-211.
- Kim H, Gallo J (2015) Evaluation of the Effect of Platelet-Rich Plasma on Recovery After Ablative Fractional Photothermolysis. JAMA Facial Plast Surg 17(2): 97-102.






























