Cutaneous Metastasis in Ovarian Serous Carcinoma- Unique Presentation at Initial Diagnosis And Review of Literature
Shereen Zia, Shannon Rodgers and Adrian Ormsby*
Department of Pathology and Lab Medicine, Henry Ford Health System, USA
Submission: January 25, 2021;Published: February 09, 2020
*Corresponding author: Adrian Ormsby, Department of Pathology and Lab Medicine, Henry Ford Health System, Detroit, USAl
How to cite this article: Shereen Z, Shannon R, Adrian O. Cutaneous Metastasis in Ovarian Serous Carcinoma- Unique Presentation at Initial Diagnosis And Review of Literature. JOJ Dermatol & Cosmet. 2021; 3(4): 555624. DOI: 10.19080/JOJDC.2021.03.555624
Abstract
Literature has shown variable morphologic presentation of cutaneous metastasis in serous ovarian cancer ,significance of time interval between diagnosis and occurrence of metastasis as well as its impact on prognosis. We present a case of a 67 years old Caucasian female who presented with an incidental finding of right lower quadrant mass, extending through the abdominal wall. Examination showed an ulcerated and vascular, right lower quadrant abdominal soft tissue mass; extending intraabdominally towards the left lower quadrant as well. CT scan demonstrated a heterogenous soft tissue pelvic mass with the largest peritoneal metastasis, eroding through the skin surface. Punch biopsy of the skin showed malignant nests of cells in the dermis. Immunohistochemical stains supported PAX-8, ER and CK7 positivity and CK20 negative expression, as well as P53 cytoplasmic staining; henceforth diagnosed as high grade serous carcinoma of ovary, involving the skin. Cutaneous metastasis in ovarian cancer is not very common, specially at the time of initial diagnosis. Our case reflects a unique and rare presentation as non-SJN metastasis at initial diagnosis.
Keywords: High grade serous carcinoma; Cutaneous metastasis; Skin biopsy
Abbreviations: HGSOC: High Grade Serous Carcinoma Of Ovary; SJN: Sister Joseph Nodule; non-SJN: Non-Sister Joseph Nodule, HPF: High Power Field
Introduction
Serous carcinoma of the ovary has been classified into a twotier system of low and high grade, of which high grade is the most common subtype. High grade serous carcinoma of ovary is an aggressive epithelial neoplasm which presents with multiple architectural patterns, (predominantly solid and papillary), highgrade cytologic atypia, marked pleomorphism and increased mitotic activity (>12 mitosis/10 high power field). Skin metastases in ovarian cancer is uncommon, present in approximately 1-6% according to published case series [1]. We report a unique report of cutaneous involvement of serous carcinoma of ovary as initial diagnostic presentation.
Case Report
We report a case of a 67 years old Caucasian female; who originally presented to the emergency department for a breast laceration and incidentally showed the providers a right lower quadrant mass extending through her abdominal wall that had developed over the last 3-4 months. The patient reported intermittent constipation that had been worsening, but otherwise denied nausea, vomiting, back pain, fatigue, hematochezia or any relevant symptoms. Physical examination revealed non-tender, non-distended abdomen with a right lower quadrant abdominal wall soft tissue mass; vascular and ulcerated in appearance, measuring approximately 4 x 5 cm; extending intraabdominally towards the spleen and left upper quadrant. There was no associated guarding or rigidity. CT abdomen/pelvis showed heterogenous soft tissue partially calcified; 7 x 7 x 10 cm pelvic mass with extensive peritoneal metastasis. The largest peritoneal mass measured approximately 24.0 x 4.5 x 5.0 cm; eroded through the skin surface within the right lower quadrant and adhered to the pelvic sidewall. A normal-appearing uterus is not visualized on imaging.
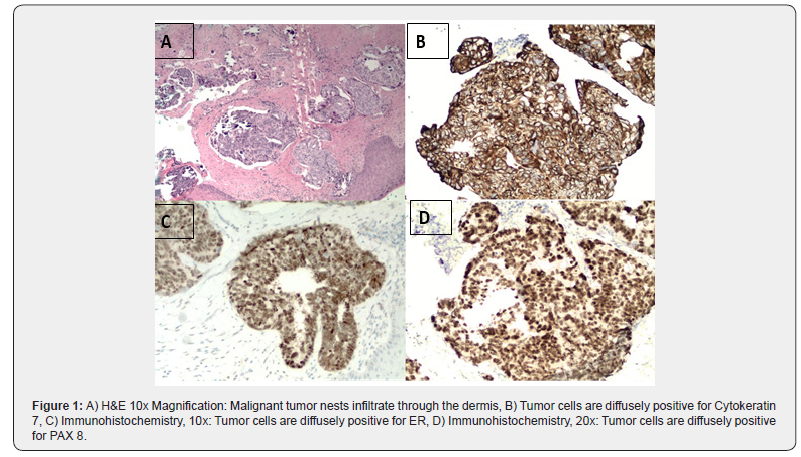
Punch biopsy of the skin invaded by the abdominal mass was taken. Further workup indicated CA-125 at 740 U/ml (Reference range: <35 U/ml). Other tumor markers were normal (CA19-9 and CEA). Histopathologic sections showed malignant nests of cells in the dermis with psammoma bodies. Immunohistochemical stains expressed positivity for Pax-8, CK7 and Estrogen receptor. CK20 immunostaining was negative (Figure 1). Tumor cells expressed cytoplasmic staining for P53 immunostaining, with wildtype nuclear staining, indicating an aberrant staining pattern. Correlation of the morphological and immunohistochemical findings supported the diagnosis of high grade serous carcinoma of müllerian origin involving the skin. Later, the patient underwent a bilateral salpingo-oophorectomy, confirming the tumor originated from the ovary. The indicated diagnosis of serous carcinoma of the ovary presenting with cutaneous metastasis is a rare occurrence. The patient has been started on neoadjuvant chemotherapy with carboplatin/paclitaxel and is being followed up.
Discussion
High grade serous carcinoma of ovary (HGSOC) is a malignant epithelial neoplasm of serous lineage; accounting for approximately 60% of all ovarian carcinomas [2]. The neoplasm has been documented to evolve from serous tubal intraepithelial carcinoma (STIC) in high-risk women carrying germline BRCA1 or 2 mutations [3] and molecular studies indicated TP53 mutations in 80% of cases as well as lack of mutations (KRAS and BRAF mutations) that are found in the low grade tumors [4]. Clinically, HGSOC is associated with an aggressive behavior, later age at diagnosis (mean age, 6th-7th decade) and poor prognosis [5]. In addition to germline BRCA1 and BRCA2 mutations, other genes related to DNA repair; like PALB2 and RAD51c have been affiliated with the carcinogenesis of this malignancy; therefore; molecular characterization has led to targeted therapeutic options for the treatment of high grade serous carcinoma [6]. Microscopically, high grade serous carcinoma is characterized by complex papillary, solid and glandular architecture; composed of pleomorphic, columnar to cuboidal cells with eosinophilic cytoplasm. The cells show marked nuclear atypia, prominent eosinophilic nucleoli and high mitotic index; generally greater than 12 mitosis/ 10 high-power fields (HPFs) [7]. It is important to differentiate high grade carcinoma from metastatic carcinomas and low-grade serous carcinoma; to determine clinical outcome and treatment modality. The presence of frank stromal invasion (>3.0 mm) differentiates low-grade serous carcinomas from serous tumors of low malignant potential (serous tumor of borderline malignancy, atypical proliferating tumors) [8]. WT1 positivity establishes the diagnosis of primary ovarian serous tumors. The immunohistochemical expression of p53 (abnormal/mutation-type pattern) is considered concordant with high-grade tumors [9].
Distant and cutaneous metastasis in epithelial ovarian cancer has been related with poor/low survival rate. A retrospective study conducted at University of Bari on 220 patients revealed median survival after diagnosis of cutaneous metastasis from epithelial ovarian cancer to be 4 months [10].It also highlighted the importance of time interval between diagnosis of ovarian cancer and occurrence of skin involvement; and correlated cutaneous metastasis with poor prognosis. Common metastatic spread in epithelial ovarian carcinoma are liver, lung and distant lymph nodes [11]. Cutaneous metastasis is uncommon [12]; and has been classified as umbilical metastasis (Sister Joseph nodule [SJN]) and nonumbilical metastasis [13]. Sister Joseph Nodules (SJN) are regarded as a manifestation of an advanced stage of the disease as well as considered to present at the time of initial diagnosis [14]. Whereas non-SJN skin metastases have been associated with scenarios of recurrent findings [15]. Amongst different involved sites in non-SJN skin metastasis; the abdominal wall is the most frequently involved and usually preceded by an incisional scar of the primary surgery [16]. Non-SJN metastasis can present variably; ranging from painless subcutaneous nodules to papules, plaques, hemangioma-like-nodules [17] and rarely, an extremely large cauliflower-type tumor [18]. Although skin metastasis has been correlated with poor prognosis; but case reports regarding patients with non SJN metastases show a favorable outcome in that comparison [19]. chemotherapy, with immune checkpoint blockade/ monoclonal antibody. Individualized management options have also been proposed.
References
- Otsuka I (2019) Cutaneous Metastases in Ovarian Cancer. Cancers (Basel) 11(9): 1292.
- Köbel M, Kalloger SE, Huntsman DG, Santos JL, Swenerton KD, et al. (2010) Cheryl Brown Ovarian Cancer Outcomes Unit of the British Columbia Cancer Agency, Vancouver BC. Differences in tumor type in low-stage versus high-stage ovarian carcinomas. Int J Gynecol Pathol 29(3): 203-211.
- Kim J, Park EY, Kim O, Schilder JM, Coffey DM, et al. (2018) Cell Origins of High-Grade Serous Ovarian Cancer. Cancers (Basel) 10(11): 433.
- Kurman RJ, Shih IeM (2010) The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory. Am J Surg Pathol 34(3): 433-443.
- Chen GM, Kannan L, Geistlinger L, Kofia V, Safikhani Z, et al. (2018) Consensus on Molecular Subtypes of High-Grade Serous Ovarian Carcinoma. Clin Cancer Res 24(20): 5037-5047.
- Kohn EC, Ivy SP (2017) Whence High-Grade Serous Ovarian Cancer. Am Soc Clin Oncol Educ Book 37: 443-448.
- Rosen DG, Zhang Z, Shan W (2010) Morphological and molecular basis of ovarian serous carcinoma. J Biomed Res 24(4): 257-263.
- McKenney JK, Balzer BL, Longacre TA (2006) Patterns of stromal invasion in ovarian serous tumors of low malignant potential (borderline tumors): a reevaluation of the concept of stromal microinvasion. Am J Surg Pathol 30(10): 1209-1221.
- Manu V, Hein TA, Boruah D, Srinivas V (2020) Serous ovarian tumors: Immunohistochemical profiling as an aid to grading and understanding tumorigenesis. Med J Armed Forces India 76(1): 30-36.
- Cormio G, Capotorto M, Di Vagno G, Cazzolla A, Carriero C, et al. (2003) Skin metastases in ovarian carcinoma: a report of nine cases and a review of the literature. Gynecol Oncol 90(3): 682-685.
- Dauplat J, Hacker NF, Nieberg RK, Berek JS, Rose TP, et al. (1987) Distant metastases in epithelial ovarian carcinoma. Cancer 60(7): 1561-1566.
- Tharakaram S (1988) Metastases to the skin. Int J Dermatol 27(4): 240-242.
- Otsuka I, Matsuura T (2017) Skin metastases in epithelial ovarian and fallopian tube carcinoma. Medicine (Baltimore) 96(33): e7798.
- Otsuka I (2019) Cutaneous Metastases in Ovarian Cancer. Cancers (Basel) 11(9): 1292.
- Yilmaz Z, Bese T, Demirkiran F, Ilvan S, Sanioglu C, et al. (2006) Skin metastasis in ovarian carcinoma. Int J Gynecol Cancer 16(S1): 414-418.
- Kadar N (1997) Port-site recurrences following laparoscopic operations for gynaecological malignancies. Br J Obstet Gynaecol 104(11): 1308-1313.
- Brownstein MH, Helwig EB (1972) Metastatic tumors of the skin. Cancer 29(5): 1298-1307.
- Traiman P, de Luca LA, Bacchi CE (1994) An extremely large, cauliflower-type, cutaneous metastasis of ovarian cancer associated with good prognosis 53(2): 239-241.
- Cheng B, Lu W, Xiaoyun W, YaXia C, Xie X (2009) Extra-abdominal metastases from epithelial ovarian carcinoma: an analysis of 20 cases. Int J Gynecol Cancer 19(4): 611-614.






























