Increased Vitamin D Signalling Markers in the Skin of Atopic Dermatitis Patients
Christin Weise1,2, Margitta Worm1 and Ralph Rühl3,4*
1Department of Dermatology and Allergology, Allergy-Center-Charité, Charité-Universitätsmedizin, Germany
2NBS Scientific GmbH, Weinheim, Germany
3Department of Biochemistry and Molecular Biology, University of Debrecen, Hungary
4Paprika Bioanalytics BT, Debrecen, Hungary
Submission: December 09, 2020;Published: December 22, 2020
*Corresponding author: Ralph Rühl, Paprika Bioanalytics BT, Debrecen, Hungary
How to cite this article: Christin W, Margitta W, Ralph R. Increased Vitamin D Signalling Markers in the Skin of Atopic Dermatitis Patients. JOJ Dermatol & Cosmet. 2020; 3(4): 555618. DOI: 10.19080/JOJDC.2020.03.555618
Abstract
Vitamin D-mediated signalling is discussed controversial in regard of allergic sensitization and atopic dermatitis (AD). In this study we determined that in AD patients two main target genes of vitamin D-mediated signalling CYP24A1 (vitamin D 24-hydroxylase) and TSLP (thymic stromal lymphopoietin) are strongly upregulated in both affected as well as non-affected skin of AD patients vs healthy volunteers skin. We conclude that this increased local vitamin D-mediated signalling resulting is in a strong local pro-inflammatory and pro-allergic conditions present in the skin of AD patients with an impact on the systemic vitamin D-signalling and systemic allergic sensitization.
Keywords:Atopic Dermatitis; Skin; Vitamin D; Thymic Stromal Lymphopoietin; Allergic Sensitization; 24-Hydroxylase
Introduction
Serum vitamin D levels have been described to be positively or negatively affected in atopic dermatitis [1-4]. Endogenous serum as well as skin concentrations of the endogenous bioactive vitamin D receptor (VDR) ligand 1,25-dihydroxy-vitamin D3 (1,25VD3) are hard to determine using analytical techniques due to the low endogenous levels in the range of 10-12M [5]. Just ELISA / RIA techniques in combination with prior chromatography enable a reliable determination of this derivative in serum. Levels of the 1,25VD3-precursor 25-hydroxy-vitamin D3 (25VD3), which is present in higher levels in serum, can routinely be determined in serum samples. Polymorphisms of the VDR are present in patients with severe AD indicating a strong association of vitamin D-mediated signaling and AD [6]. In addition the VDR can hetero-dimerize with the retinoid X receptor (RXR) which ligands [7-9] were associated with positively influencing VDR-RXR-mediated signalling [10,11].
In various studies reduced serum levels of 25VD3 are found in serum of adult and young AD patients and were even partly inversely correlating with the disease status [12-14]. Unfortunately it is not clear if this phenomenon is due to reduced dietary vitamin D intakes, reduced UV skin exposure and/or targeted down- regulation of serum vitamin D homeostasis via vitamin D binding proteins [15,16], comparable to fatty acids and retinoids under inflammatory conditions, reviewed in Rühl [17,18]. Positive effects of vitamin D supplementation or administration of more stable and less calcemic synthetic VDR agonists are found on clinical markers of AD [19,20], while other studies determined negative effects [21,22]. Various studies postulate that due to reduced 25VD3 levels targeted vitamin D supplementation may be an optimal treatment strategy for improving AD conditions [23,24]. Unfortunately, just limited AD-relevant target organs like the skin and the immune system were examined for vitamin D-mediated signaling during allergic sensitization, chronic manifested atopic dermatitis and after vitamin D-supplementation studies [12-14]. These lacks of knowledge make it difficult to judge the potential positive and negative impact of vitamin D signaling and supplementation in AD.
Case Presentation
In this study we determined based on immunohistological studies the semi-quantitative occurrence of the two VDR-signaling markers TSLP and CYP24A1 [22,25] in normal skin from healthy volunteers compared to affected and non-affected skin of AD-patients. CYP24A1, the 24-hydroxylase responsible for inactivation of 1,25VD3, is the most common and sensitive marker of VDR-mediated signaling [25], while TSLP is a wellknown vitamin D target and trigger of TH2-signalling and thereby a major initiator of allergic sensitization [22,26,27]. For both proteins an increased presence was observed in the epidermis and infiltrating cells in dermis of affected and non-affected skin of ADpatients compared to skin of healthy volunteers (Figure 1). ADskin possesses remarkably more CYP24A1 protein in epidermis, especially in the stratum basale. CYP24A1 expressing cells were broadly distributed throughout the dermis of healthy and AD-skin. Interestingly, the number of CYP24A1 expressing infiltrating cells seems to be smaller in both affected and non-affected AD-skin than in healthy skin. In contrast to CYP24A1, TSLP presence is scattered in the epidermis of healthy skin and strongly increased in differentiating epidermal keratinocytes over the stratum basale of AD-skin. Furthermore, the proportion of infiltrating cells with TSLP presence is further increased in affected skin, thereby increasing TSLP levels in AD-lesions.
Discussion
Our data describe a strongly induced vitamin D signaling and thereby pro-allergic conditions in the affected and non-affected skin of AD-patients. These data are partly in contrast with the described reduced 25VD3 serum values present in AD-patients and the potential connection of reduced vitamin D intake, represented by reduced 25VD3 serum levels, and AD [1,14,28,29]. We suggest that serum vitamin D levels may be reduced by our organisms in a feedback mechanism to dampen local vitamin D–mediated proallergic and pro-inflammatory conditions, comparable to feedback mechanisms of serum vitamin A levels during local inflammation [17,18,30].
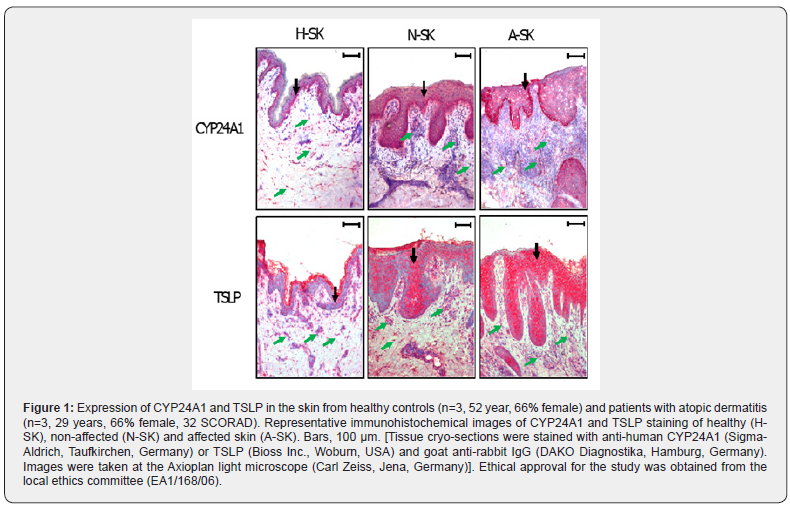
Conclusion
These results prove that vitamin D mediated signaling is strongly increased in affected and non-affected skin of AD-patients and thereby resulting in strong pro-inflammatory and pro-allergic conditions present in the skin of atopic dermatitis
References
- Albert PJ, Proal AD, Marshall TG (2009) Vitamin D: the alternative hypothesis. Autoimmun Rev 8(8):639-644.
- Wjst M (2006) The vitamin D slant on allergy. Pediatr Allergy Immunol 17(7):477-83.
- Wjst M (2012) Is vitamin D supplementation responsible for the allergy pandemic? Curr Opin Allergy Clin Immunol 12(3):257-62.
- Lucas R, Mihaly J, Gericke J, Torocsik D, Ruhl R (2020) Vitamin D signaling in a mouse allergic sensitization model. Int J Vitam Nutr Res 90(5-6):385-8.
- Lyra EC, Silva IA, Katayama ML, Brentani MM, Nonogaki S, et al. (2006) 25(OH)D3 and 1,25(OH)2D3 serum concentration and breast tissue expression of 1alpha-hydroxylase, 24-hydroxylase and Vitamin D receptor in women with and without breast cancer. J Steroid Biochem Mol Biol 100(4-5):184-192.
- Heine G, Hoefer N, Franke A, Nothling U, Schumann RR, et al. (2013) Association of vitamin D receptor gene polymorphisms with severe atopic dermatitis in adults. Br J Dermatol 168(4):855-888.
- Krezel W, Ruhl R, Lera AR (2019) Alternative retinoid X receptor (RXR) ligands. Mol Cell Endocrinol 491:110436.
- Rühl R, Krzyzosiak A, NiewiadomskaCA, Rochel N, Szeles L, et al. (2015) 9-cis-13,14-dihydroretinoic acid is an endogenous retinoid acting as RXR ligand in mice. PLoS Genet 11(6):e1005213.
- Rühl R, Krezel W, Lera AR (2018) 9-Cis-13,14-dihydroretinoic acid, a new endogenous mammalian ligand of retinoid X receptor and the active ligand of a potential new vitamin A category: vitamin A5. Nutr Rev 76(12):929-41.
- Treptow S, Grun J, Scholz J, Radbruch A, Heine G, et al. (2020) 9-cis retinoic acid and 1.25-dihydroxyvitamin D3 drive differentiation into IgA(+) secreting plasmablasts in human naive B cells. Eur J Immunol.
- Li XY, Xiao JH, Feng X, Qin L, Voorhees JJ (1997) Retinoid X receptor-specific ligands synergistically upregulate 1, 25-dihydroxyvitamin D3-dependent transcription in epidermal keratinocytes in vitro and in vivo. J Invest Dermatol 108(4):506-512.
- Brehm JM, Celedon JC, SotoQME, Avila L, Hunninghake GM, et al. (2009) Serum vitamin D levels and markers of severity of childhood asthma in Costa Rica. Am J Respir Crit Care Med 179(9):765-771.
- Peroni DG, Piacentini GL, Cametti E, Chinellato I, Boner AL (2011) Correlation between serum 25-hydroxyvitamin D levels and severity of atopic dermatitis in children. Br J Dermatol 164:1078-1082.
- Weisse K, Winkler S, Hirche F, Herberth G, Hinz D, et al. (2013) Maternal and newborn vitamin D status and its impact on food allergy development in the German LINA cohort study. Allergy 68(2):220-228.
- Touvier M, Deschasaux M, Montourcy M, Sutton A, Charnaux N, et al. (2014) Determinants of Vitamin D Status in Caucasian Adults: Influence of Sun Exposure, Dietary Intake, Sociodemographic, Lifestyle, Anthropometric, and Genetic Factors. J Invest Dermatol 135(2): 378-388.
- Bratke K, Wendt A, Garbe K, Kuepper M, Julius P, et al. (2014) Vitamin D binding protein and vitamin D in human allergen-induced endobronchial inflammation. Clin Exp Immunol 177(1):366-372.
- Rühl R (2013) Non-pro-vitamin A and pro-vitamin A carotenoids in atopy development. Int Arch Allergy Appl Immunol 161:99-115.
- Rühl R (2007) Effects of dietary retinoids and carotenoids on immune development. Proc Nutr Soc 66(3):458-69.
- Hartmann B, Riedel R, Jorss K, Loddenkemper C, Steinmeyer A, et al. (2012) Vitamin D receptor activation improves allergen-triggered eczema in mice. J Invest Dermatol 132(2):330-336.
- Hartmann B, Heine G, Babina M, Steinmeyer A, Zugel U, et al. (2011) Targeting the vitamin D receptor inhibits the B cell-dependent allergic immune response. Allergy 66(4):540-548.
- Back O, Blomquist HK, Hernell O, Stenberg B (2009) Does vitamin D intake during infancy promote the development of atopic allergy? Acta Derm Venereol 89(1):28-32.
- Li M, Hener P, Zhang Z, Kato S, Metzger D, et al. (2006) Topical vitamin D3 and low-calcemic analogs induce thymic stromal lymphopoietin in mouse keratinocytes and trigger an atopic dermatitis. Proc Natl Acad Sci U S A 103(31):11736-41.
- Peroni DG, Boner AL (2013) Food allergy: the perspectives of prevention using vitamin D. Curr Opin Allergy Clin Immunol 13(3):287-292.
- Devereux G, Macdonald H, Hawrylowicz C (2009) Vitamin D and asthma: time for intervention? Am J Respir Crit Care Med 179(9):739-740.
- Prosser DE, Jones G (2004) Enzymes involved in the activation and inactivation of vitamin D. Trends Biochem Sci 29(12):664-673.
- Jessup HK, Brewer AW, Omori M, Rickel EA, Budelsky AL, et al. (2008) Intradermal administration of thymic stromal lymphopoietin induces a T cell- and eosinophil-dependent systemic Th2 inflammatory response. J Immunol 181(6):4311-4319.
- Soumelis V, Liu YJ (2004) Human thymic stromal lymphopoietin: a novel epithelial cell-derived cytokine and a potential key player in the induction of allergic inflammation. Springer Semin Immunopathol 25(3-4):325-333.
- Searing DA, Leung DY (2010) Vitamin D in atopic dermatitis, asthma and allergic diseases. Immunol Allergy Clin North Am 30(3):397-409.
- Muehleisen B, Gallo RL (2013) Vitamin D in allergic disease: shedding light on a complex problem. J Allergy Clin Immunol 131(2):324-329.
- Gieng SH, Raila J, Rosales FJ (2005) Accumulation of retinol in the liver after prolonged hyporetinolemia in the vitamin A-sufficient rat. J Lipid Res 46(4):641-649.






























