Periorbital Vitiligo Caused by Wrinkle-Removing Eye Cream: A Case Report
Arthur E Brawer*
Department of Medicine, Division of rheumatology, Monmouth Medical Center, New Jersey, USA
Submission: November 11, 2019; Published: November 20, 2019
*Corresponding author: Arthur E Brawer, Department of Medicine, Division of rheumatology, Monmouth Medical Center, New Jersey, USA
How to cite this article: Arthur E Brawer. Periorbital Vitiligo Caused by Wrinkle-Removing Eye Cream: A Case Report. JOJ Dermatol & Cosmet. 2019; 2(1): 555578.
Abstract
The prospect of slowing and/or reversing the aging process of one’s skin by applying topical over-the-counter commercial products is becoming increasingly attractive. In particular, facial wrinkle removers are generating significant consumer attention, but the potential for these products to cause permanent skin damage has not been previously addressed. This report investigates the ingredients found in a single product and correlates their presence with an adverse clinical outcome.
Keywords: Periorbital Vitiligo Commerical Products Wrinkle Removers Detergents Adhesives Motor Oils Skin Pigmentation Eye Cream
Introduction
Chemical induced vitiligo usually occurs following skin exposure to phenol containing compounds that may be unknowingly present in worksite industrial materials, hair dyes, skin lightening creams, household cleaning products, deodorants, detergents, adhesives, rubber gloves and sandals, motor oils, and laboratory reagents [1,2]. It is clinically indistinguishable from spontaneous loss of skin pigmentation related to genetic risks and autoimmune phenomena. We report a case of periorbital vitiligo caused by a routine commercially available eye cream advertised to remove unwanted wrinkles. None of the risk factors mentioned above are relevant to this case.
Case Report
A 51-year-old Caucasian female applied Lancome Renergie Lift Multi-Action Eye Cream twice a day for one week in the following locations: below her eyebrows, underneath her lower eyelids, and the lateral corners of her eyes. There was no previous history utilizing similar products, and there was no history of burning, itching, nor skin rash in the application contact areas. On the eighth day she ceased usage after noticing progressive loss of pigmentation in the areas of application (Figures 1-3). Over the next three years her periorbital vitiligo remained unimproved despite a variety of therapeutic dermatologic interventions, including laser treatments. No other areas of skin depigmentation have developed. Her only other ongoing medical problem was a four-year history of mild, non-progressive, untreated multiple sclerosis characterized by intermittent fatigue, self-limited lower extremity myalgias, occasional lack of coordination, and occasional memory lapses
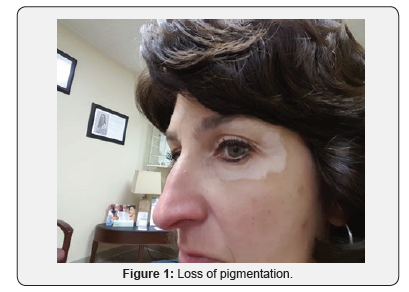
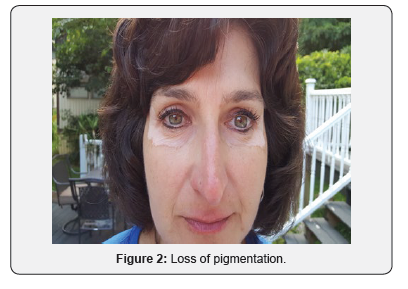
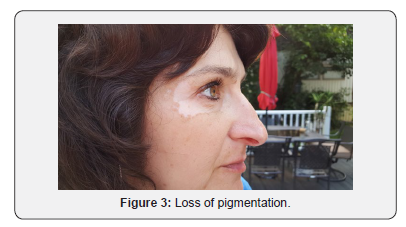
Discussion
There exists an exhaustive list of ingredients in the topical product used by this patient, namely: water, dimethicone, glycerin, isohexadecane, alcohol, squalene, cetyl alcohol, stearic acid, mineral oil, palmitic acid, PEG-100 stearate, glycerol stearate, PEG-20 stearate, beeswax, octyldodecanol, Ci77891, titanium dioxide, Ci16035 (red 40), Ci19140 (yellow 5), C13-14 isoparaffin, mica, saccharomyces (yeast), hydrolyzed linseed extract, sodium hydroxide, soy protein, hyalauronic acid, sodium benzoate, phenoxyethanol, adenosine, acetyl tetrapeptide, caffeine, silica, polyacrylamide, chlorphenasin, chlorhexidine digluconate, polyethylene dimethiconol, limonene, benzyl alcohol, linalool, capryloyl, salicylic acid, microcrystalline wax, paraffin, shea butter, laureth-7, coumarin, and parfum. Dimethicone and dimethiconol are polydimethylsiloxanes (silicones), which chemically are the same artificial organosiloxane materials present in silicone gel-filled breast implants. These two items, in concert with octyldodecanol (an emulsifier), readily penetrate the skin and adhere to the subcutaneous fat, as silicones are lipophilic. Fat cells (adipocytes and lipocytes) contain a number of mediators of inflammation, and fat cells are also capable of converting the amino acid tyrosine into catecholamines (epinephrine and norepinephrine). Tyrosine is an essential ingredient used by melanocytes to produce normal skin pigmentation (melanogenesis). Vitiligo and tachyarrythmias are two items in a long list of proven toxic clinical phenomena manifested by recipients of silicone gel-filled breast implants [3-5].
It is therefore hypothesized that one of the mechanisms of vitiligo production in this patient is the localized preferential shunting of tyrosine usage to activated adipocytes, thereby depriving melanocytes of their substrate. A second mechanism is silicone-induced stimulation of adipocytes to produce localized inflammation, as panniculitis (subcutaneous fat necrosis) is another well-known ailment occurring in implant recipients [3-5]. Localized inflammation has been shown to be lethal to melanocytes [1,2]. Five other ingredients in this topical product are also of critical importance in the development of vitiligo in this patient. Two of these include salicylic acid and sodium benzoate. Salicylic acid by itself acts as an exfoliant to reduce dry patches, and sodium benzoate by itself is designed to kill bacteria on the skin. The other three key ingredients are sodium hydroxide, calcium hydroxide (present in mica), and water. When all five are mixed togethera chemical reaction ensues between salicylic acid and sodium benzoate, whereby phenols are produced. Phenols are directly toxic to melanocytes because they act as tyrosine analogues, thereby inhibiting the process of melanogenesis [1,2].
All of the other ingredients in this product have varied actions. For example, isohexadecane in an emollient and a solvent designed to soften and smooth the skin in concert with enabling other substances to easily dissolve into a homogeneous entity. Ci16035 (red 40) is known to cause allergic reactions, and Ci19140 (yellow 5) is known to cause purple spots on the skin. Squalene is utilized for adding moisture but is known to clog pores in the skin. Phenoxyethanol is a preservative known to cause skin irritation. Silica has a long and sordid history of toxicity to the body. Polyacrylamide absorbs water and is used as a thickener. Chlorphenesin is utilized as a preservative and is known to cause contact dermatitis. Chlorhexidine digluconate is an antiseptic utilized to kill bacteria. Limonene is an antioxidant and also is known to cause skin senisitivity. Laureth-7 is a surfactant that contains 1,4-dioxane (a solvent that is a known toxin). Essentially, Lancome Renergie Lift Multi-Action Eye Cream is a soup mixture of ingredients with the clear-cut potential to damage the skin via many adverse mechanisms. Over the past ten years the proliferation of over-the-counter anti-aging materials are legion, and the same is true for multiple other multi-purpose topical products such as moisturizers, softeners, rejuvenators, make-up, lotions, shampoos, and soaps. One can only speculate how many other consumers may have developed permanent vitiligo upon exposure to similar compounds encountered in this case, since many or all of these compounds are not necessarily restricted to just wrinkle removers.
With regard to cosmetics, using a make-up remover at the end of the day may paradoxically enhance an adverse skin reaction, because many removers contain an emulsifier known as TEA (triethanolamine). TEA temporarily changes the pH of the skin to alkaline, thereby transiently loosening the tight bonds between skin cells. Thus, a certain proportion of what is on the skin may actually wind up being impacted below it. The induction of wrinkle remover induced vitiligo may be more likely to occur if the user does not experience skin burning, itching, and or rashes on initial contact. Under these circumstances (as noted in this case) a consumer is more likely to continue further applications beyond the first day or two of usage. Once subclinical inflammation and melanocyte damage become obvious, it is probably too late to reverse the unsightly cosmetic outcome.
Conclusion
Consumers need to be aware of the complexity of topical skin products they are purchasing, as these products may contain potentially harmful ingredients capable of causing unwanted and permanently altered dermatologic appearances.
References
- Harris JE (2017) Chemical-induced vitiligo. Dermatologic Clinics 35(2): 151-161.
- Rietschel RL, Fowler JF, Fisher AA(2008) Fisher’s Contact Dermatitis, sixth edition. PMPH-USA 2008; chapter 38: Contact Vitiligo, pp. 675-685.
- Brawer AE(1996) Chronology of systemic disease development in 300 symptomatic recipients of silicone gel-filled breast implants. J Clean Technol, Environ Toxicol, Occup Med 5(3): 223-233.
- Brawer AE (2017) Destiny rides again: the reappearance of silicone gel-filled breast implant toxicity. Lupus 26(10): 1060-1063.
- Brawer AE (2017)Mechanisms of breast implant toxicity: will the real ringmaster please stand up. In Annals Med 11(9): 249.






























