Serum Levels of 25-Oh Vitamin D in Psoriatic Patients and Control Subjects
Michelle dos Santos Diniz*, Jackson Machado Pinto and Maria Marta Sarquis Soares
Department of Medicine, University of Federal Minas Gerais, Brazil
Submission: March 01, 2019; Published: March 25, 2019
*Corresponding author: Michelle dos Santos Diniz, Department of medicine, University of Federal Minas Gerais, Brazil
Michelle dos Santos Diniz, Jackson Machado Pinto, Maria Marta Sarquis Soares. Serum Levels of 25-Oh Vitamin D in Psoriatic Patients and Control Subjects. JOJ Dermatol & Cosmet. 2019; 1(4): 555567.
Abstract
Background: Literature data on the association of psoriasis with vitamin D deficiency are still controversial.
Aim: To compare serum levels of vitamin D of adults with psoriasis without arthritis and controls.
Materials and Methods: The analysis of vitamin D serum levels was performed by chemiluminescent immune-assay. Statistical data were evaluated by the tests: Fisher’s Exact, Student’s t and Mann-Whitney; the values were considered significant when P< 0.05.
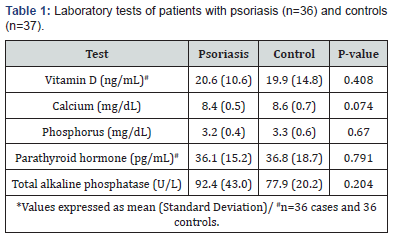
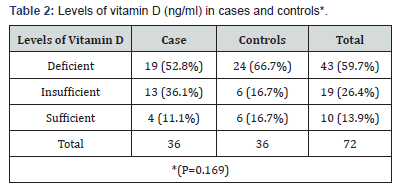
Results: The study included 36 patients with psoriasis, mean age was 45.8±14.4 years; 21 (58.3%) patients were male. The control group comprised 37 participants with a mean age of 40.3±12.1 years; 20 (54.1%) of these were male. There was no difference between the groups considering sex (P=.815) and age (P =.088). BMI for the psoriasis and control groups were 27.2±4.1kg/m2 and 27.2±4.8kg/m2 (P=0.921), respectively. The laboratory tests showed no significant difference between the groups for vitamin D (p=0.408), calcium (p=0.074), phosphorus (p=0.670), parathyroid hormone (0.791), total alkaline phosphatase (0.204). Most cases (52.8%) and controls (66.7%) had deficient levels of vitamin D. Insufficient levels vitamin D were found in 36.1% of cases and 16.7% of controls. Adequate levels of vitamin D were found in 11.1% of psoriatic patients and 16.7% of controls. There was no statistically significant difference between the groups (p=0.169).
Conclusion: The exact role of vitamin D in psoriasis need to be further studied. We emphasize the high prevalence of inadequate vitamin D levels in both groups.
Keywords: Psoriasis Vitamina D Psoriatic Arthritis
Introduction
Psoriasis is a chronic inflammatory autoimmune disease that affects about 1-3% of the world population [1,2]. Several comorbidities have been associated with psoriasis as obesity, diabetes mellitus, hypertension. More recently some studies showed a possible association with low bone mineral density [3]. Literature data on the association of psoriasis with vitamin D deficiency are still controversial [4]. Vitamin D is considered the oldest hormone on earth and its main source comes from the exposure of the skin to ultraviolet B. In addition to the already well-established benefit in calcium homeostasis, it is known that the potential actions of vitamin D is very broad. Vitamin D receptors have been identified in several cells as in the enterocytes, osteoblasts, immune cells, parathyroid cells, keratinocytes and ovarian cells [4]. This study compares serum levels of vitamin D of adults with active psoriasis without arthritis and patients seen at the outpatient dermatology clinic.
Materials and Methods
We studied adult patients with a clinical and/or histological diagnosis of psoriasis without joint complaints who were seenat the Dermatology Outpatient Clinic of the Santa Casa de Belo Horizonte Hospital, Brazil. All the patients were evaluated by a professional experienced in psoriasis diagnosis and treatment. The exclusion criteria were postmenopausal women; men older than 70 years of age; smoking, alcohol dependence; presence of other chronic inflammatory diseases; a report or confirmed diagnosis of neoplasia or HIV infection; use of systemic retinoid in the previous 12 months; current use of systemic glucocorticoids, insulin, statins, growth hormone (GH), anabolic agents, or hormone replacement therapy; pregnancy or lactation. The control group included voluntary patients without psoriasis or other inflammatory diseases, matched for sex and age, who received care at the Dermatology Outpatient Clinic of the Santa Casa de Belo Horizonte Hospital.
The data were collected during a routine visit for psoriasis follow-up at the Dermatology Outpatient Clinic of the Santa Casa de Belo Horizonte Hospital. The demographic and anthropometric data were obtained from interviews and medical examinations. Blood samples for analysis were collected after eight hours of fasting.
The analysis of vitamin D serum levels was performed by chemiluminescent immune-assay and values <20 ng/ml were considered deficiency, from 20 to<30ng/ml insufficiency and ≥30ng/ml sufficiency. Statistical data were evaluated by the tests: Fisher’s Exact, Student’s t and Mann-Whitney; the values were considered significant when P<0.05.
Results
In the period of May through November 2012, the study included 36 patients with psoriasis without joint complaints. The patients’ mean age was 45.8±14.4 years; 21 (58.3%) of the patients were male. The mean reported duration of disease was 12.6±9 years. Twenty-two (61.1%) patients were receiving systemic treatment and three (8.6%) patients had PASI (Psoriasis Area and Severity Index) ≥10 which is considered a moderate to severe disease. The control group comprised 37 participants with a mean age of 40.3±12.1 years; 20 (54.1%) of these were male. There was no difference between the groups with respect to sex (P=.815) and age (P=.088). The values of BMI for the psoriasis and control groups were 27.2±4.1kg/m2 and 27.2 ± 4.8kg/m2 (P = 0.921), respectively.
The laboratory tests showed no significant difference between the groups for vitamin D (p=0.408), calcium (p=0.074), phosphorus (p=0.670), parathyroid hormone (0.791), total alkaline phosphatase (0.204) (Table 1). Most cases (52.8%) and controls (66.7%) had deficient levels of vitamin D. Insufficient vitamin D levels were found in 36.1% of cases and 16.7% of controls. Adequate levels of vitamin D were found in 11.1% of psoriatic patients and 16.7% of controls. There was no statistically significant difference between the groups for vitamin D levels (p=0.169) (Table 2).
Discussion
Vitamin D has a number of functions in the skin inhibits proliferation and promotes the differentiation of keratinocytes, modulates the humoral and cellular immune system and participates in the hair cycle. These actions are due to the binding of vitamin D in their receptors present in keratinocytes. These cells in turn are themselves capable of producing vitamin D which acts on receptors in an autocrine action. The importance of vitamin D in psoriasis can be demonstrated by a good therapeutic response to vitamin D analogues used topically in the treatment of this disease such as calcipotriol. Recent studies have shown that vitamin D can act on the immune system by inhibiting important cytokines for Th1 and Th17 differentiation that are one of the most important pathways in the pathophysiology of psoriasis [4-6]. However, what is not clear yet is whether serum vitamin D or vitamin D locally produced by keratinocytes themselves is the responsible for these effects. There are few randomized controlled studies on the effect of oral supplementation of vitamin D in psoriasis and none was able to show real benefit [7].
In this research, no difference was found in the concentrations of vitamin D between the patients with psoriasis and controls. The literature is controversial regarding the greater prevalence of vitamin D deficiency in patients with psoriasis. Patients with psoriasis were believed to have higher levels of vitamin D because they are frequently instructed to have greater sun exposure, since the lesions respond well to ultraviolet radiation. Nonetheless, some studies have shown that vitamin D levels may be actually lower in this population in particular, whether due to impracticability of sun exposure or fear of exposing the lesions in public, or even as a result of keratinocyte dysfunction and inability to initiate adequate production of vitamin D in the skin. Three studies involving 43, 46, and 145 patients with psoriasis found significantly lower concentrations of vitamin D in the patients with psoriasis compared to controls [8-10]. Vitamin D participates in the keratinocyte growth and differentiation process and also seems to influence the immune function of dendritic cells and T-lymphocytes, being capable of inhibiting the production of IL-2 and IL6, which would account for the fact that vitamin D levels are altered in patients with psoriasis [11]. However, a large population-based study with 148 cases of psoriasis and 5,693 controls found no difference in vitamin D concentrations between the groups [10]. Solak et al. [12] studied a quite similar population studied in this research and also found no statistically significant difference in the levels of vitamin D compared to controls. A recent Brazilian study also failed to demonstrate difference in the vitamin D levels between psoriasis patients and controls [13] as well as Cubillos et al. [7] in Germany.
Another important aspect of the results that should be noted is that the average values of vitamin D found both in patients (20.6ng/ml) and controls (19.9ng /ml) are on the boundary between the levels considered deficient and insufficient according to the Brazilian and American societies of endocrinology. Only11.1% of cases and 16.7% of controls had vitamin D levels above 30ng/ml which is the level considered sufficient. Zuchi et al. [13] found similar values in another Brazilian state: 10% of cases and 20% of controls had sufficient levels of vitamin D demonstrating the high prevalence of deficient /insufficient levels not only in patients with psoriasis but also in the controls. These data are consistent with other research in the country that found average levels of Vitamin D of 21.4% with a prevalence of deficiency above 77% [14]. The fact that the population of this study show high BMI can justify an even higher prevalence of deficient /insufficient levels of vitamin D since obesity tend to be associated with lower levels of this vitamin due to the storage of the fat-soluble vitamin in the fatty tissue. Different from other studies, this study evaluated not only vitamin D but also other markers of bone metabolism such as calcium, phosphorus, parathyroid hormone and alkaline phosphatase. In both groups, cases and controls, we did not observe changes in these dosages. This may suggest that psoriasis does not seem to be a major factor in calcium and vitamin D metabolism. It is noteworthy that even with low levels of vitamin D, elevation of parathyroid hormone was not observed.
We can highlight some limitations of the study as the small number of people studied so that we can’t make inferences for the entire psoriatic population. In addition, the dosage of vitamin D was taken over eight months what may undergo seasonal variations. This fact was offset by the inclusion of both cases and controls throughout the period.
Conclusion
In conclusion, the exact role of vitamin D in psoriasis needs to be further studied. Although we could demonstrate low levels of vitamin D on psoriatic patients, it was not different than what we found in the group taken as control. It is possible that, for these patients, the low serum level of vitamin D could reflect in the local action of vitamin D in keratinocytes which must be discarded in other studies. Furthermore, there was no difference in calcium metabolism in patients with psoriasis and patients with other skin diseases. We emphasize the high prevalence of inadequate vitamin D levels in both groups.
Acknowledgement
FAPEMIG -Foundation for the Support of Research State of Minas Gerais
Conflict of Interest
This research received support and funding from the Foundation for the Support of Research State of Minas Gerais (FAPEMIG) and Janssen-Cilag Pharmaceutical.
References
- Duarte VG, Porto Silva L, Oliveira MPF (2015) Epidemiology and treatment of psoriasis: a Brazilian perspective. Psoriasis: Targets and Therapy 5: 55-64.
- Menegon D, Pereira AG, Camerin AC, Cestari TB (2014) Psoriasis and comorbidities in a southern Brazilian population: a case-control study. Int J Dermatol 53(11): e518-e525.
- Machado Pinto J, Diniz MS, Bavoso NC (2016) Psoriasis: new comorbidities. A Bras Dermatol 91(1): 8-14.
- Soleymani T, Hung T, Soung J (2015) The role of vitamin D in psoriasis: a review. Int J Dermatol 54(4): 383-392.
- Mattozzi C, Paolino G, Richetta AG, Calvieri S (2016) Psoriasis, vitamin D and the importance of the cutaneous barrier’s integrity: An update. J Dermatol 43(5): 507-514.
- Piotrowska A, Wierzbicka J, Żmijewski MA (2016) Vitamin D in the skin physiology and pathology. Acta Biochimica Polonica 63(1): 17-29.
- Cubillos S, Krieg N, Norgauer J (2016) Effect of Vitamin D on Peripheral Blood Mononuclear Cells from Patients with Psoriasis Vulgaris and Psoriatic Arthritis. PLoS ONE 11(4): e0153094.
- Atwa MA, Balata MG, Hussein AM, Abdelrahman NI, Elminshawy HH (2013) Serum 25-hydroxyvitamin D concentration in patients with psoriasis and rheumatoid arthritis and its association with disease activity and serum tumor necrosis factor-alpha. Saudi Med J 34(8): 806-813.
- Gisondi P, Rossini M, Di Cesare A, Idolazzi L, Farina S, et al. (2012) Vitamin D status in patients with chronic plaque psoriasis. Br J Dermatol 166(3): 505-510.
- Wilson PB (2013) Serum 25-hydroxyvitamin D status in individuals with psoriasis in the general population. Endocrine 44(2): 537-539.
- Schwalfenberg GK (2011) A review of the critical role of vitamin D in the functioning of the immune system and the clinical implications of vitamin D deficiency. Mol Nutr Food Res 55(1): 96-108.
- Solak B, Dikicier BS, Celik HD, Erdem T (2016) Bone Mineral Density, 25-OH Vitamin D and Inflammation in Patients with Psoriasis. Photodermatol Photoimmunol Photomed 32(3): 153-160.
- Zuchi MF, Azevedo PO, Tanaka AA, Schmitt JV (2015) Martins LEAM. Serum levels of 25-hydroxy vitamin D in psoriatic patients (2015) An Bras Dermatol 90(3): 430-432.
- Unger MD (2009) Determinação dos níveis séricos de vitamina D em uma amostra de indivíduos saudáveis da população brasileira, São Paulo. Tese (Doutorado em Nefrologia) Universidade de São Paulo.






























