Abstract
Rowell syndrome is a combination of erythema multiforme lesions, and systemic lupus erythematosus. We report this case of male patient, 15 years old, presented to the emergency department with fever, and skin rash. This fever started two weeks ago after he received triple therapy for Helicobacter pylori treatment. The fever was associated with generalized fatigability, and arthralgia in both knee joints. The skin rash was erythema multiforme major like. His laboratory results showed pancytopenia, positive immunological tests of speckled Antinuclear Antibody (ANA), Anti-Double Stranded DNA Antibodies (Anti-dsDNA), anti -RO, anti -La antibodies, and rheumatoid factors. The skin, lips, oral, and tongue lesions were improved after treatment of 40 mg once daily of prednisolone tablet, but, unfortunately, the patient had Deep Venous Thrombosis (DVT) in the right dorsalis pedis due to secondary development of antiphospholipid syndrome after few days of admission. The antiphospholipid syndrome was associated with high coagulation profile, thrombocytopenia. positive tests of Anti- Beta 2 glycoprotein IgG, and Lupus Anticoagulant 1 with high Lupus ratio of Lupus Anticoagulant 1, and Lupus Anticoagulant 2 (LA1/LA2).
Keywords: Rowell syndrome; Antiphospholipid syndrome; Lupus anticoagulant; Anticardiolipin antibodies; Antinuclear antibody
Abbreviations: ANA: Antinuclear Antibody; Anti-dsDNA: Anti-Double Stranded DNA Antibodies; DVT: Deep Venous Thrombosis; LA1/LA2: Lupus Anticoagulant 1, and Lupus Anticoagulant 2; WBC: White Blood Cell Count; RBC: Red Blood Cell Count; PTT: Partial Thromboplastin Time; SLE: Systemic Lupus Erythematosus; aPLs: Antiphospholipid Antibodies; TIAs: Transient Ischemic Attacks; APS: Antiphospholipid Syndrome
Introduction
Male patient, 15 years old, presented to the emergency department with fever, and skin rash which was erythema multiforme major like. This fever started 2weeks ago after he received triple therapy for H. pylori treatment. The fever was associated with generalized fatigability, and arthralgia in both knee joints. He had a history of dactylitis in some hand fingers, some toes.
On examination
The patient was febrile with temperature of 38.7ºC, conscious, oriented, both knee joints were tender without swelling. The other joints were free, mild pericardial effusion. There was no dactylitis. The other systems were free.
On the dermatological examinations
There was an acute onset of skin rash which was presented in the sun exposed areas like the face, v- shaped area of the neck and front of chest. The lesions were presented in the form of erythematous macular rash with target lesions in the center. There were erosions in the lips, tongue, and hard palate with bleeding from lips. There was no bleeding from other sites. There were no bullous lessons. The extremities and trunk were free from the lesions except the front of the chest. There was no chilblain, discoid lupus lesions and Raynaud’s phenomenon. His calf muscle was normal in consistency, soft, and without discoloration. The skin, lips, oral mucosa, and tongue lesions were improved after treatment with 40 mg once daily of prednisolone tablets, with supportive treatment to his high coagulation profile, and plaquenil 400 mg per day to control lupus erythematosus activity. Unfortunately, the patient had DVT in the right dorsalis pedis due to secondary development of antiphospholipid syndrome after few days of admission which was associated with oedema and tender with hotness in the right calf muscle, high coagulation profile, thrombocytopenia, positive tests of Anti- Beta 2 glycoprotein IgG, and Lupus Anticoagulant 1 with high Lupus ratio of LA1/LA2.
Laboratory Investigations
As regards his complete blood count
The clinically significant results are as follows:
White Blood Cell Count (WBC) of 2.33k/μL (L) (n 4-10k/ μL), with a neutrophilic count of 1.54k/μL (L) (n 2-7.5k/μL), Red Blood Cell Count (RBC) of 2.66M/μL (L) (n 4.5-5.5M/μL) with a hemoglobin of 78gm/L (L), hematocrit of 0.20L/L (L) (n 0.39- 0.54), Platelet count of 161K/μL (N) (n 150-410K/μL), and ESR OF 116 ml/h (H) (n 0-10ml/h).
As regards his chemistry
His random glucose of 6.20mmol/L (N) (n 4.11-8.33mmol/L), high blood urea of 8.4mmol/L (n 2.76-8.7mmol/L), high creatinine of 112 μmol/L (n 62-106μmol/L), high SGOT (AST) (85U/L) (n 0-35U/L), high SGPT (ALT) (45U/L) (H) (n 0-26U/ L), normal total bilirubin (7μmol/L) (n 0-21μmol/L), normal direct bilirubin (3.10μmol/L) (n 0-3.4μmol/L), low albumin (28.40g/L) (n 39.7-49.4g/dL), low chloride (94.90mmol/L) (n 98-107mmol/L), low sodium (123mmol/L) (n 135-151mmol/L), critical high level of ferritin 3739ng/mL (30-400ng/mL), low iron of 4.4μmol/L (n 5.83-34.50μmol/L), low calcium of 2.04mmol/L (2.1-2.55mmol/L), high lipase of 147U/L (n 13-60U/L), normal potassium (4.33mmol/L) (n 3.4-5.1mmol/L), normal alkaline phosphatase of 50U/L (n 0-390U/L), and normal magnesium of 0.71mmol/L (n 0.7-0.91mmol/L),
As regards coagulation profile
Partial Thromboplastin Time (PTT) was (74.30sec.) (high) (n 26-40 seconds), Thromboplastin Time (PT) of (21.60sec.) (high) (n 11.7-15.3 seconds), and INR of 1.59 % (high) (n 0.8-1.2%).
As regards serology
Rheumatoid factor was positive of 36U/mL (n. < 20U/mL). ANA (Antinuclear Antibody) Elisa was 500U/ml (positive). Anti- ds DNA antibodies (Anti-Double Stranded DNA Test) was also positive. Direct Coombs test was positive, but indirect is negative. Anti- Beta 2 glycoprotein IgG was positive, but Anticardiolipin IgM is negative. Lupus Anticoagulant 1 was high at level of 111.5 seconds (n. ≤ 43 seconds). Lupus Anticoagulant 2 was normal at level of 0sec (n. ≤ 35 seconds). Lupus ratio LA1/LA2 was 1.85 which was high (n. ≤1.30).
As regards the urine examination
There were Proteinuria of 0.17g/L (n. 0.01-014g/L).
Echocardiogram showed mild pericardial effusion
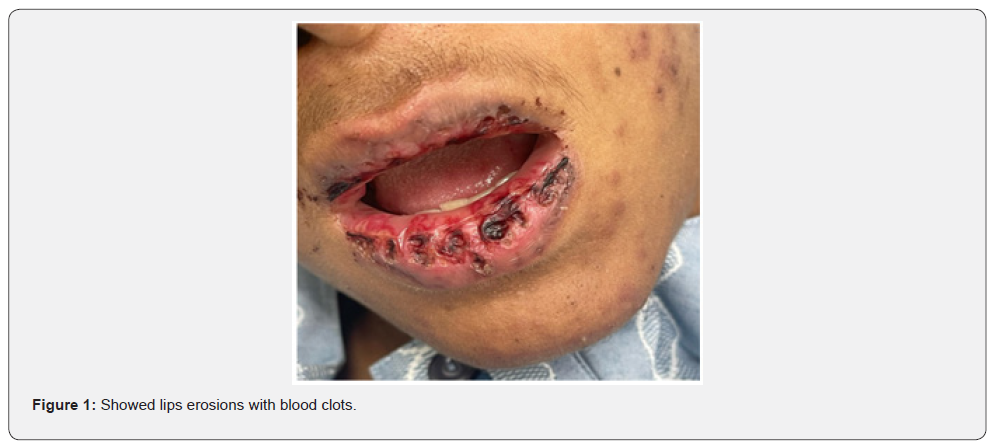
Discussion
Rowell Syndrome is a rare dermatological condition characterized by a combination of skin manifestations associated with Systemic Lupus Erythematosus (SLE) and erythema multiforme. Patients with Rowell Syndrome typically exhibit distinctive skin lesions, including a butterfly-shaped rash across the cheeks and nose, like that seen in lupus, as well as target lesions of erythema multiforme [1,2]. This syndrome is often classified under the umbrella of lupus erythematosus, highlighting its connection to autoimmune processes that affect the skin and other organs [3]. The condition was first described in medical literature in the mid- 20th century, with Dr. Rowell being among the first to identify the unique combination of symptoms that characterize this syndrome [1]. Since its initial discovery, the understanding of Rowell Syndrome has evolved, yet it remains a topic of ongoing research and clinical interest due to its complex interplay with other autoimmune disorders [4].
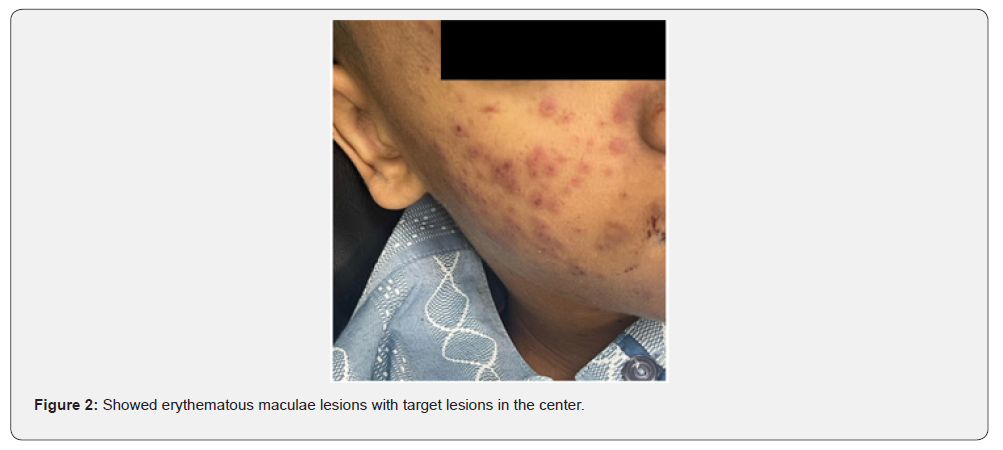
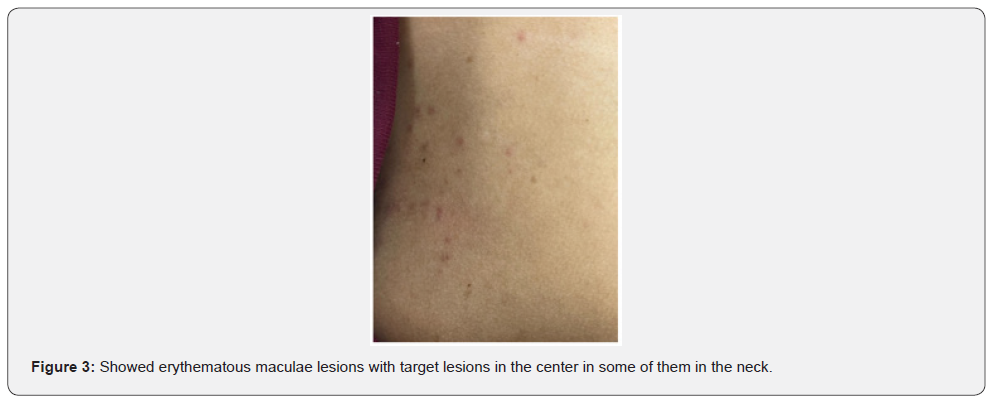
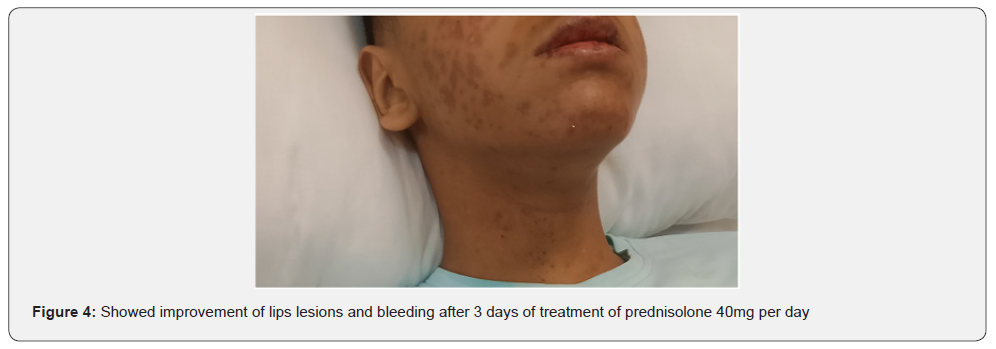
Studying Rowell Syndrome is crucial for several reasons. First, it enhances our understanding of the pathophysiology of autoimmune diseases, particularly how they manifest in the skin [5]. Second, it raises awareness among healthcare professionals, which is vital for accurate diagnosis and effective management of affected individuals [2]. Furthermore, the study of Rowell Syndrome contributes to the broader understanding of the pathophysiological mechanisms underlying autoimmune reactions. Research has highlighted the role of immunological factors and genetic predispositions in the development of this syndrome, shedding light on the complexities of autoimmunity in dermatological contexts. By exploring these connections, researchers can identify potential biomarkers and therapeutic targets that may benefit not only those with Rowell Syndrome but also individuals suffering from related autoimmune conditions [5]. Additionally, raising awareness of Rowell Syndrome among healthcare providers is crucial for improving patient outcomes. Misdiagnosis or delayed diagnosis can lead to inappropriate treatments, exacerbating the patient’s condition [2].
Following its initial description, Rowell Syndrome garnered attention in the dermatological community as researchers began to explore its clinical implications. The connection between Rowell Syndrome and other autoimmune diseases became a focal point for subsequent studies, as it was recognized that the syndrome could serve as an indicator of underlying lupus erythematosus [3]. Over the years, various case studies and reviews have contributed to a broader understanding of the syndrome, including its epidemiology, pathophysiology, and potential treatment strategies [2,3]. Continued research about Rowell Syndrome may lead to the development of more effective therapeutic protocols, improving the quality of life for affected individuals. As studies reveal new insights into the syndrome, they may also inform treatment guidelines for other autoimmune diseases, ultimately contributing to a more comprehensive approach to patient care [3].
The speckled pattern of the ANA is the immunological characteristic most consistent of RS, appearing in 60.4% to of the cases, although it is not exclusive of this condition [6]. Therapy should be initiated with corticosteroid and hydroxychloroquine. In some cases, success was obtained with dapsone, cyclosporine or azathioprine [7,8].
Antiphospholipid Syndrome (APS) is an acquired, autoimmune thrombophilia in the presence of persistent Antiphospholipid Antibodies (Apls) [9,10]. Antiphospholipid Syndrome (APS) encompasses antibodies to phospholipids/phospholipid-binding cofactor proteins and/or circulating lupus anticoagulant with clinical manifestations of thrombosis such as recurrent spontaneous abortions [11]. APS typically manifests as major arterial and/or venous thrombosis and/or obstetric morbidity; and it is these features that form the major classification criteria for APS [12].
APS has several other recognized clinical manifestations, such as livedo reticularis, cutaneous necrosis, thrombocytopenia hemolytic anemia, neuropsychiatric phenomena (e.g., Transient Ischemic Attacks (TIAs), cognitive deficits or white matter lesions), cardiac valve disease and nephropathy [10]. Pandhi et al. [13] reported a case of primigravida with systemic lupus erythematosus, erythema multiforme-like lesions and a peculiar immunological pattern consisting of antinuclear antibody (speckled pattern) and rheumatoid factor, an association known as Rowell’s syndrome. She also had probable antiphospholipid syndrome as evidenced by the presence of a prolonged activated partial thromboplastin time, kaolin clotting time and thrombocytopenia.
Conclusion
We report a case of Rowell syndrome with secondary development of antiphospholipid syndrome. This rare case presented with overlapping clinical picture of systemic lupus erythematosus, erythema multiforme, and antiphospholipid syndrome. It is essential to workup by dermatologists, rheumatologists and primary care physicians to reach the diagnosis, management, and control of pathogenesis and symptoms of these complexities of autoimmune syndrome. They must also make every effort to prevent the complications of this syndrome and improve the quality of life.
References
- Rowell NR (1963) A case of lupus erythematosus and erythema multiforme. Archives of Dermatology 88(3): 314-319.
- Toole OA, Mendez J, Grice, J (2018) A rare case of Rowell syndrome in a middle-aged patient presenting with erythematous skin lesions. British Journal of Dermatology 178(2): 545-547.
- Dinehart SM, Garlipp MA (2015) Autoimmune skin disorders: An overview. Dermatologic Clinics 33(4): 611-623.
- Brunetti AE, Bianchi M, Marra C (2017) Rowell syndrome: A comprehensive review of its clinical features and management. Journal of Dermatology 44(3): 304-310.
- Katz SI, Glick BM, Heller MM (2019) Autoimmunity and the skin: The role of Rowell syndrome. International Journal of Dermatology 58(5): 577-584.
- Antiga E, Caproni M, Bonciani D, Bonciolini V, Fabbri P (2012) The last word on the so-called Rowell's syndrome. Lupus 21(6): 577-585.
- Cornelia SLM, Louisa R, Vogt T (2011) Successful treatment of Rowell syndrome using oral cyclosporine A. Int J Dermatol 50(8): 1020-1022.
- Bhobe MR, Tambe S, Jindal S, Jerajani HR (2015) Rowell's syndrome to ds-DNA negative lupus nephritis: a yet unreported progression. Indian J Dermatol 60(2): 215.
- Garcia D, Erkan D (2018) Diagnosis and Management of the antiphospholipid syndrome. N Engl J Med 378(21): 2010-2021.
- Uthman I, Noureldine MHA, Ruiz-Irastorza G, Khamashta M (2019) Management of antiphospholipid syndrome. Ann Rheum Dis 78(2): 155-161.
- Sharma YK, Chauhan S (2018) Overlap Syndrome with Rowell's Syndrome, Antiphospholipid Syndrome, Primary Sterility, and Sensorineural Hearing Loss: A Case Report, Brief Review, and Analysis of Cases Indian and Abroad. Indian J Dermatol 63(5): 418-23.
- Miyakis S, Lockshin MD, Atsumi T, Branch DW, Brey RL, et al. (2006) International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 4(2): 295-306.
- Pandhi D, Singal A, Agarwal P (2004) Rowell syndrome and associated antiphospholipid syndrome. Clin Exp Dermatol 29(1): 22‑2






























