Abstract
Radiographic evidence of fusion is the primary outcome measure for most spinal fusion clinical studies. While X-rays and computed tomography (CT) scans reveal radiopaque (radiodense) matter in the fusion mass, which is often defined as bridging bone and residual graft material, additional methods are essential to clarify the presence of mature bone. The tissue and cellular organization within a spinal fusion site can only be assessed with histology, which is routinely performed in pre-clinical animal studies. Because histological evidence of fusion in humans is difficult to obtain as it involves taking a biopsy of the patient’s fusion mass, there is a paucity of histological evidence of fusion in humans. This case report summarizes results from a revision spinal fusion surgery at twenty-two months after the primary surgery with post-operative radiographic analysis as well as histological assessment of biopsies of the fusion mass. The bone graft used in the case was MagnetOs Putty. This is the first human case report to highlight histological evidence of large amounts of bone formation following spinal fusion surgery with MagnetOs Putty. The histologic evidence of bone formation is corroborated by radiographic observations.
Keywords: Radiographic evidence; Bridging bone; MagnetOs Putty; Bone grafts; Orthopedic and neurosurgery fusion; Biopsies; Histology; Bone marrow
Introduction
The gold standard with which all other bone graft materials are compared remains autologous cancellous bone harvested from the iliac crest. It is osteoconductive, osteoinductive, contains bone progenitor cells, and provides structural support [1]. However, there is also morbidity associated with its harvest. Thirty nine percent of patients may complain of chronic pain at the donor site after iliac crest harvest [2]. In addition, there is added operative time and blood loss and a possible risk of infection due to this second operation [3]. Further, the volume of cancellous bone that can be harvested is limited [4]. The limited availability of autologous bone as well as the donor site morbidity associated with iliac crest grafting necessitates the development of alternative grafts with comparable clinical outcomes [5].
Allogenic bone obtained from human donors or cadavers has been used as an alternative to autograft. Allograft bone circumvents the morbidity associated with iliac crest bone harvest. However, limitation in allograft supply, risk of immunogenic response, and potential for disease transmission provide limitations of allograft [6]. Procedures for minimizing the risk of an immunogenic response and disease transmission may alter the biologic and mechanical properties; allograft processed by freezing and irradiation limits disease transmission but reduces the osteoinductive properties of the bone compared to the initial bone tissue. Despite the extent of processing and sterilization, the risk for disease transmission cannot be eliminated [7]. Another disadvantage of allograft is its variable quality between donors, lots, and manufacturing sites [8]. The relative concerns over use of either autograft or allograft have led to the development of numerous bone graft substitutes [9].
To alleviate the need for patient-derived autograft or cadaver-derived allograft, advanced surface topography bone grafts were developed to promote bone formation through mechanical stimulation of bone-building cells [10]. Calcium phosphates (CaPs) are synthetic bone graft substitutes that are used for orthopedic and neurosurgery fusion indications. The chemical composition of CaPs resembles that of native bone mineral, which makes them osteoconductive. While most conventional CaPs are merely osteoconductive, a subset of CaPs have the ability to trigger de novo bone formation in sites far from host bone [10- 13]. Pre-clinical studies reveal that CaPs with submicron surface topography are more effective than CaPs without submicron topography at forming bone through the core of the bone graft [10,11,14]. MagnetOs bone grafts are CaPs with advanced submicron surface topography that result in accelerated bone formation and demonstrated equivalency to autograft in clinically relevant animal models [15-17]*. Furthermore, MagnetOs Granules demonstrated noninferiority and even “indicated superiority” compared to autograft in a posterolateral fusion human clinical study [18]. MagnetOs Putty is composed of 95% by volume of MagnetOs Granules and 5% of a LEOL polymer (polyethylene glycol and polylactic acid, PEG/PLA), to facilitate handling and placement of the bone graft.
Histology provides insight into the tissue architecture and cellular organization of bone at a single time point after surgery. Histological analysis assists with discerning residual bone graft material compared to new bone formation, which can be difficult to distinguish in radiographs. Instrumented posterolateral fusion studies in ovine and lapine models reveal histological evidence of bone formation with MagnetOs [16,17]. Furthermore, canine paraspinal muscle pouch histology demonstrates the ability of MagnetOs to grow bone through the core of the bone graft, in the absence of added cells, proteins, or pre-existing bone [15]*^. However, few human studies evaluate histological evidence of bone growth in response to bone grafts in humans. One case report in a complicated foot and ankle revision surgery revealed bone formation at 12 weeks post-operatively with MagnetOs bone graft [19]. The following case report is the first to document histological evidence of bone formation in response to MagnetOs in posterolateral fusion, twenty-two months postoperatively.
Materials and Methods
The study involves a prospective collection of remnant tissue biopsies from a revision surgery at twenty-two months post-operative in a patient who underwent a posterolateral fusion surgery with MagnetOs Putty used standalone without additional bone graft. Per protocol, the patient was required to meet all inclusion criteria and not meet any of the exclusion criteria to participate in the study (Table 1).
After institutional review board permission was obtained, potential patients for study inclusion were identified from electronic medical records. Once identified, research staff reviewed a clinical consent form with the patient to enroll the indicated patient in the study. Remnant patient tissue was obtained during the revision surgery and processed according to the following fixation, decalcification, and processing protocol compatible with Mechano-Therapeutics, LLC (MT) processing and staining protocols. Three remnant tissue samples from distinct areas were collected. Tissue samples were at least 1mm in diameter and 1-5mm in length. The remnant tissue samples were immediately placed in tissue fixative (4% formalin or 1.5% glutaraldehyde) and stored at 4°C for 4 days. The samples were then rinsed and placed in 70% reagent alcohol (RA) at room temperature. The fixed remnant tissue was decalcified, embedded in a paraffin block, sectioned, and scanned based on protocols designed and optimized by MT. Briefly, specimens were washed once with distilled water (DW) and decalcified in a Formical-2000 decalcifier (StatLab) at 20x the volume of each sample, with daily changes of solution for 12 days. Specimen were rinsed to remove residual decalcification and placed in 70% RA. Each sample was embedded in paraffin and sectioned at 5μm thickness at the pathology core of the Children’s Hospital of Philadelphia (CHOP). The sections were stained with Hematoxylin and Eosin (H&E) using standard protocols and digitally scanned using an Aperio Scanscope CS-O digital slide scanner at 20x magnification. For trichrome staining, briefly, slides were deparaffinized with sequential washes in Citrisolv hybrid (Deacon labs) and 70-100% RA, followed by water. They were then stained with Weigert’s hematoxylin for 10 min, washed with running tap water to develop the dye, then in 0.05% Fast Green FCF in DW for 20 min, followed by 0.1% sirius red in saturated picric acid for 30 min. The samples were washed with two changes in 1% glacial acetic acid in water. They were then dehydrated with reverse washes going from 70% RA to Citrosolv hybrid and mounted with cover glass for imaging. Stained slides were imaged on a Nikon TS2 Eclipse microscope at 4x magnification, using a Digital Sight 1000 camera (Nikon). The primary endpoint was histological analysis of remnant tissue during a revision surgery with MagnetOs Putty bone graft at least 18 months following the primary fusion procedure. Secondary outcome measures include radiographic analysis of pathology and fusion at 12 months and 22 months post-operatively.
Ethical Conduct
This clinical study was conducted in accordance with the protocol, the ethical principles that have their origin in the Declaration of Helsinki, and Good Clinical Practice and applicable regulatory requirements including IRB review and approval.
Results
Patient demographics and surgical information
A 26-year-old female patient underwent a thoracolumbar fusion, T3-L1 with 40cc of MagnetOs Putty used standalone on one side (right) of the construct to correct a sagittal spinal curve. A mixture of other bone grafts was used on the left side of the posterolateral spine. Twenty-two months post-operatively, the patient presented with persistent muscle spasms. Radiographic evidence of fusion was visible. The patient was scheduled for a revision surgery to remove the hardware. The patient did not have any notable prior medical history, prior surgical history, or comorbidities.
Radiographic outcomes
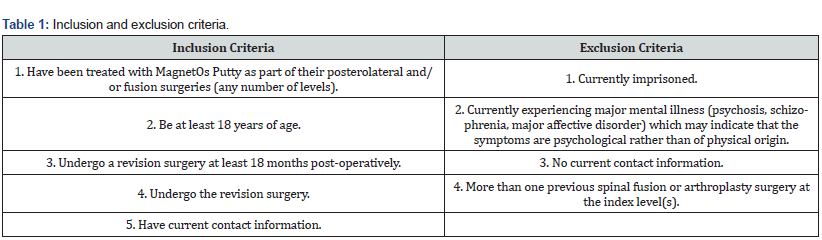
X-rays were obtained 12 months following the primary T11-L1 surgery. The X-rays revealed maintained placement of hardware and satisfactory spinal alignment. MagnetOs Putty appears consolidated, with evidence of a smooth cortical ring around the bone formed (Figure 1).
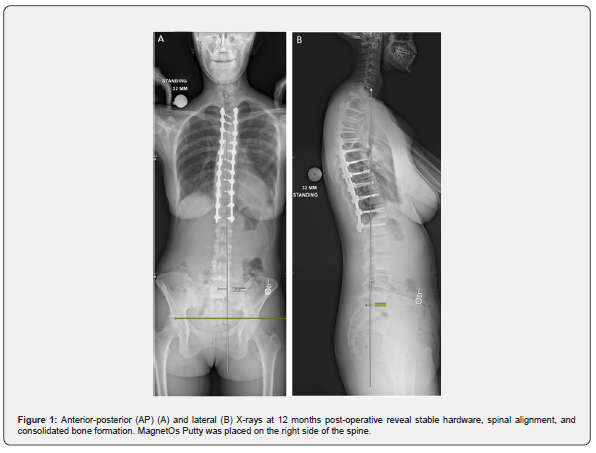
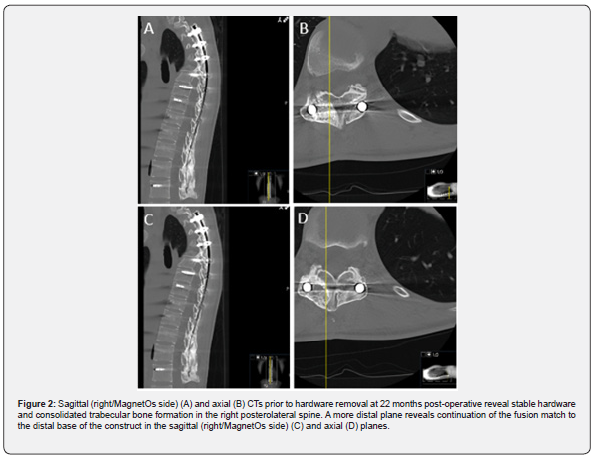
Twenty-two months post-operatively the patient presented with muscle spasms. Computed tomography (CT) revealed maintained hardware alignment and trabecular bone on the right side of the posterolateral spine (Figure 2). The bone formation remained evident immediately post-hardware removal (Figure 3).
Histological assessment
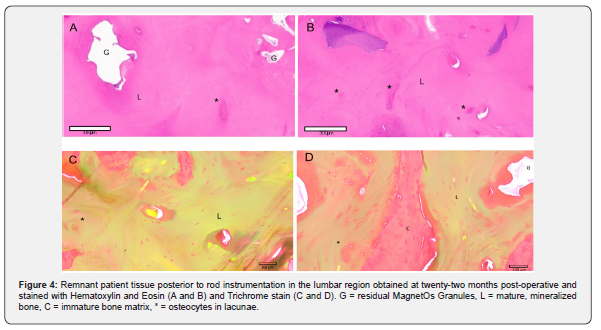
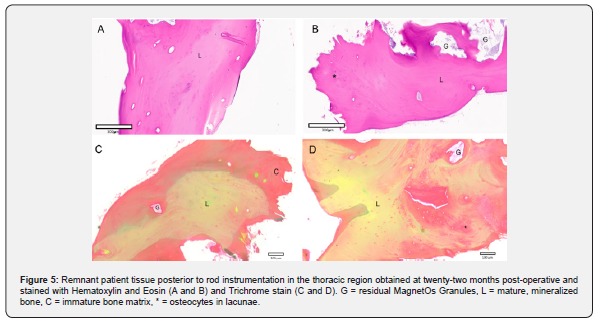
Biopsies were obtained from the side of the fusion with MagnetOs Putty used standalone without additional bone graft material. During removal of the rod that was placed between the pedicle screws at the lumbar and thoracic regions of the spine, biopsy tissue present on top of the rod was obtained and analyzed. Representative histological sections were evaluated. Histological analysis of the lumbar region revealed immature and mature mineralized bone matrix and some empty decalcified regions representing remnant MagnetOs Granules embedded within the healthy bone structure (Figure 4). No signs of fibrous tissue were observed in the fusion mass. Likewise, biopsy tissue obtained from on top of the rods in the thoracic section revealed large amounts of healthy immature and mineralized bone with some empty decalcified regions representing MagnetOs Granules implant remnants (Figure 5).
Discussion
Patient reported outcomes, radiographs, and histology can all be used to assess spinal fusion surgery outcomes. Patient reported outcomes directly assess patient pain and quality of life, while radiographs and histology provide insight into the extent of bone formation. X-rays and CT scans can be taken over multiple time points and are non-invasive. Limitations of X-rays and CTs include that it may be difficult to distinguish newly formed bone from remnant bone graft in the radiopaque image, while the resolution is also limited. Histology requires tissue resection, which is invasive, while it only captures a single plane at a single time point. However, histology clarifies distinct tissue types (e.g., bone graft, bone, and fibrous tissue) and the resolution provides insight into the integration of different tissues and cellular organization. Pairing histological data with radiographic outcomes can clarify the tissue composition in a fusion mass while also providing quantitative measures for bone volume. For example, an ovine study comparing MagnetOs, VitossTM (tricalcium phosphate), NovaboneTM (Bioglass), and autograft used manual palpation, radiology (X-ray and microcomputed tomography), and histology to evaluate fusion outcomes [20]. The radiographs and microCT images were graded on the Lenke scale. Levels with a radiographic fusion grade B with Novabone did not contain any bone tissue and revealed evidence of remnant bone graft material when evaluated via histology. This demonstrates the unique insight provided by histology that reveals the cellular interactions with bone grafts that are involved with material resorption, inflammation, and bone deposition. It also shows the limitation of radiology for determining bony fusion.
This case report correlated radiographic X-ray and CT observations with histological evidence. X-rays obtained at 12 months post-operatively and CT scans at 22 months post-operatively revealed spongy trabecular bone in multiple planes. Post-operative complications requiring hardware removal provided an opportunity to study a biopsy at the site of the fusion. Histology further supported the formation of healthy bone at 22 months. Residual MagnetOs Granules are evident but well-incorporated into lamellar bone at 22 weeks. Resorption of MagnetOs Granules occurs at a physiological rate, with evidence of some bone graft expected at 22 weeks. The adult skeleton is resorbed and remodeled approximatively every 10 years [21].
There have been few studies that have histologically assessed fusion outcomes with bone grafts following posterolateral spinal fusion surgery in humans. Existing histological evidence of bone formation with bone grafts in spinal fusion surgery is limited to the intervertebral disc space with cages and autogenous bone graft [22,23]. One cohort study analyzed autograft bone incorporation into cages in nine radiographically fused patients. The tissue was obtained via needle biopsy from the core of the intervertebral disc space and was analyzed 8-72 months post-operatively (mean 28 months). Fragments of necrotic bone associated with viable bone and hematopoietic bone marrow in varied ratios among the nine patients [22]. Another study used histology to evaluate the percent bone ingrowth in eleven radiographically or clinically failed interbody fusions with titanium cages. The cages were removed 2-47 months post-operatively and all revealed evidence of vascular ingrowth and areas of histologically viable bone. Debris were present in 9/11 cages, fibrocartilage composed 0-70% of the cage space, and the average viable bone area was 31% [23]. Both histologic reports from spinal fusion used autologous bone and examined bone growth in the intervertebral space. There are no studies to date that evaluate histological evidence of bone formation with a synthetic bone graft for posterolateral fusion.
A foot and ankle case report revealed the ability of bone to grow in and on MagnetOs [19]. The case report documents a 73-year-old female patient with an ankle fracture dislocation that was fused with MagnetOs. The patient had multiple comorbidities, including neuropathy, diabetes mellitus type II, peripheral artery disease, hypertension, and hyperlipidemia. Twelve weeks after the tibiotalar arthrodesis, the patient presented with Charcot arthropathy and underwent a revision. During the revision, remnant tissue was obtained and processed for histology. The histology revealed the MagnetOs bone graft integrated well into the fusion site, with bone growing in and around the graft [19].
This case report is the first to evaluate the histology of a remnant patient tissue biopsy from a posterolateral spinal fusion surgery with MagnetOs. Future studies aim to evaluate histological evidence of bone formation with MagnetOs used for other spinal indications in a diverse demographic population and at various time points.
Conclusion
The case presented in this report provides radiographic and histological evidence of bone formation in and on MagnetOs bone graft following a posterolateral spinal fusion surgery. Large amounts of healthy immature and mature mineralized bone were apparent in the lumbar and thoracic regions after a T3-L1 fusion, twenty-two months post-operatively. Remnant MagnetOs Granules were seen embedded and incorporated within the bony fusion mass. This is the first case report to demonstrate robust bone formation after spinal fusion surgery with an advanced synthetic bone graft, assessed via a combination of radiographic and histological methods.
Acknowledgementa
Mechano-Therapeutics LLC completed the tissue processing, sectioning, and staining.
*Results from in-vivo or in-vitro laboratory testing may not be predictive of clinical experience in humans.
^MagnetOs is not cleared by FDA or TGA as an osteoinductive bone graft. MagnetOs is CE Marked as osteoinductive.
References
- Lane JM, Tomin E, Bostrom MP (1999) Biosynthetic Bone Grafting. Clin Ortho Relat Res (367 Suppl): S107-S117.
- Arrington ED, Smith WJ, Chambers HG, Bucknell AL, Davino NA (1996) Complications of Iliac Crest Bone Graft Harvesting. Clin Orthop Relat Res 329: 300-209.
- Sasso RC, LeHuec JC, Shaffrey C (2005) Iliac Crest Bone Graft Donor Site Pain After Anterior Lumbar Interbody Fusion: A Prospective Patient Satisfaction Outcome Assessment. J Spinal Disord Tech 18 Suppl: S77-S81.
- Hall MB, Vallerand WP, Thompson D, Hartley G (1991) Comparative Anatomic Study of Anterior and Posterior Iliac Crests as Donor Sites. J Ortal Maxillofac Surg 49(6): 560-563.
- Putzier M, Strube P, Funk JF, Gross C, Monig HJ, et al. (2009) Allogenic Versus Autologous Cancellous Bone in Lumbar Segmental Spondylodesis: A Randomized Prospective Study. Eur Spine J 18(5): 687-695.
- Mroz TE, Joyce MJ, Lieberman IH, Steinmetz MP, Benzel EC, et al. (2009) The Use of Allograft Bone in Spine Surgery: Is it Safe? Spine J 9(4): 303-308.
- Boyce T, Edwards J, Scarborough N (1999) Allograft Bone. The Influence of Processing on Safety and Performance. Orthop Clin North Am 30(4): 571-581.
- Hartigan BJ, Cohen MS (2005) Use of Bone Graft Substitutes and Bioactive Materials in Treatment of Distal Radius Fractures. Hand Clin 21(3): 449-454.
- Bauer TW, Muschler GF (2000) Bone Graft Materials. An Overview of the Basic Science. Clin Orthop Relat Res (371): 10-27.
- Yuan H, Li Y, de Bruijn JD, de Groot K, Zhang X (2000) Tissue Responses of Calcium Phosphate Cement: A Study in Dogs. Biomaterials 21(12): 1283-1290.
- Duan R, Barbieri D, Luo X, Weng J, de Bruijn JD, et al. (2016) Submicron-surface Structured Tricalcium Phosphate Ceramic Enhances the Bone Regeneration in Canine Spine Environment. J Orthop Res 34(11): 1865-1873.
- Yuan H, Yang Z, Li Y, Zhang X, de Bruijn JD, et al. (1998) Osteoinduction by Calcium Phosphate Biomaterials. J Mater Sci Mater Med 9(12): 723-726.
- Yuan H, Kurashina K, de Bruijn JD, Li Y, de Groot K, et al. (1999) A Preliminary Study on Osteoinduction of Two Kinds of Calcium Phosphate Ceramics. Biomaterials 20(19): 1799-1809.
- Davison NL, Luo X, Schoenmaker T, Everts V, Yuan H, et al. (2014) Submicron-scale Surface Architecture of Tricalcium Phosphate Directs Osteogenesis In Vitro and In Vivo. Eur Cell Mater 27: 281-297.
- Duan R, van Dijk LA, Barbieri D, de Groot F, Yuan H, et al. (2019) Accelerated Bone Formation by Biphasic Calcium Phosphate with a Novel Sub-micron Surface Topography. Eur Cell Mater 37: 60-73.
- van Dijk LA, Barbieri D, Barrere-de Groot F, Yuan H, Oliver R, et al. (2019) Efficacy of a Synthetic Calcium Phosphate with Submicron Surface Topography as Autograft Extender in Lapine Posterolateral Spinal Fusion. J Biomed Mater Res B Appl Mater 107(6): 2080-2090.
- van Dijk LA, Duan R, Luo X, Barbieri D, Pelletier M, et al. (2018) Biphasic Calcium Phosphate with Submicron Surface Topography in an Ovine Model of Instrumented Posterolateral Spinal Fusion. JOR Spine 1(4): e1039.
- Stempels HW, Lehr AM, Delawi D, Hoebink EA, Wiljouw IA, et al. (2024) Efficacy of Biphasic Calcium Phosphate Ceramic with a Needle-Shaped Surface Topography Versus Autograft in Instrumented Posterolateral Spinal Fusion: A Randomized Trial. Spine 49(19): 1323-1331.
- Fusco T, Sage K, Rush S, Blom F, Colvin K (2022) Arthrodesis of the subtalar joint using a novel biphasic calcium phosphate bone graft. FASTRAC 2(1): 100150.
- van Dijk LA, Barrere-de Groot F, Rosenberg AJWP, Pelletier M, Christou C, et al. (2020) MagnetOs, Vitoss, and Novabone in a Multi-endpoint Study of Posterolateral Fusion. Clin Spine Surg 33(6): E276-E287.
- Langdahl B, Ferrari S, Dempster DW (2016) Bone modeling and remodeling: potential as therapeutic targets for treatment of osteoporosis. Ther Adv Musculoskelet Dis 8(6): 225-235.
- Togawa D, Bauer TW, Brantigan JW, Lowergy GL (2021) Bone graft incorporation in radiographically successful human intervertebral body fusion cages. Spine 26(24): 2744-2755.
- Togawa D, Bauer T, Lieberman IH, Lowery GL, Takikawa S (2003) Histology of Tissues Within Retrieved Human Titanium Mesh Cages. Spine 28(3): 246-253.






























