Abstract
Introduction: Rivaroxaban is a new oral anticoagulant that rarely causes severe bleeding. But when spontaneous splenic rupture occurs in a patient on treatment, the drug cause and its interactions should be evaluated.
Patients and methods: We report an emergency splenectomy in a 65-year-old patient with spontaneous splenic rupture and hemodynamic destabilization. The event occurs one year after the introduction of rivaroxaban and amiodarone for persistent atrial fibrillation after cardioversion.
Results: We found and described the only 6 cases reported in literature, only one had combined rivaroxaban and amiodarone treatment.
Conclusion: Spontaneous splenic rupture is a rare event that may be precipitated by the combination of rivaroxaban and amiodarone. But there is still a lack of clinical data on this drug interaction. All potentially drug-related adverse events should be reported to the national database.
Keywords: Rivaroxaban; Splenic rupture; Amiodarone; Drug interaction
Introduction
Rivaroxaban is a direct oral anticoagulant increasingly used in routine practice. Its use ranges from primary prevention of deep vein thrombosis to treatment of pulmonary embolism and long-term prevention of thromboembolic events in patients with atrial fibrillation [1]. These new direct anticoagulant drugs are associated with an increased risk of bleeding, both traumatic and non-traumatic. Their metabolism involves the CYP3A4 isoenzyme that is functionally influenced by many drugs and [2].
Case Description
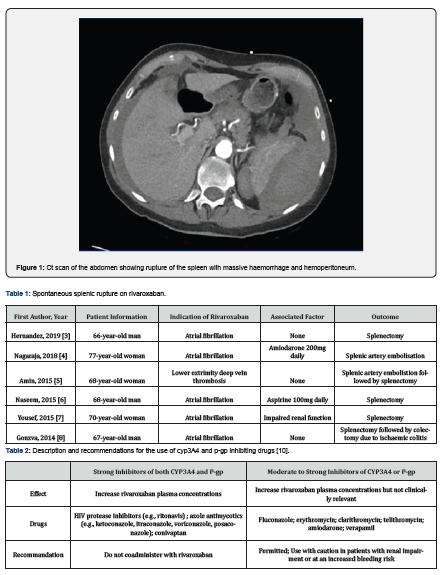
A 65-year-old woman was admitted to the emergency department by ambulance for pain in her left shoulder radiating to her left arm and back. She was dyspneic and in a presyncopal state. The pain also extended to the left hypochondrium and was dependent on breathing. The symptoms developed suddenly, without a history of trauma, infection, abdominal pain, weight loss or fever. Her history included left breast neoplasia operated on and followed by adjuvant chemoradiotherapy, gastroesophageal reflux, psoriasis, hypertension, and atrial fibrillation with impaired cardiac function. She had a sleeve gastrectomy followed by a cholecystectomy 5 years ago. Her home medications included rivaroxaban 20mg daily in the morning, pantomed 20mg daily, cordarone 200mg daily in the morning, aldactone 25mg daily, tritace 2.5mg, simvastatin 20mg, L-thyroxine 25 microgram, ledertrexate 20mg, and folavit the day after ledertrexate. On admission, her temperature was 36°C, blood pressure was 95/65mmHg, heart rate was 83/min and pulse oxymetry was 99%. She was pale and clammy, and her abdomen was diffusely painful with left hypochondrial guarding. Coagulation panel revealed international normalized ratio (INR) of 1.9 (reference range: 0.8 - 1.2), prothrombin time (PT) of 41% (reference range: 70-100%), activated cephalin time 36.1s (reference range: 25.7 - 35.9s) and platelets 272,000/µL (reference range: 150,000 - 450,000/µL). Serologic testing showed no significant abnormalities, her creatinine was 1.02mg/dl, which corresponds to a creatinine clearance of 64ml/min (according to cockroft) and her hemoglobin was 13.1g/dl (reference range: 12.0 - 16.0g/dl) on arrival in the emergency room. Two hours later hemoglobin dropped to 7.5g/dl. This drop was accompanied by progressive arterial hypotension. Computed tomography was performed before and after contrast injection and showed spontaneous splenic rupture with active hemorrhage and massive hemoperitoneum. We immediately started resuscitation with blood transfusion and fluid replacement and an emergency splenectomy was performed by median laparotomy. At the beginning of the procedure, 1.5g of tranexamic acid was administered intravenously. Approximately 3 liters of blood was aspirated from the abdominal cavity and recycled via the CellSaver. After removal of the spleen, examination of the abdominal cavity did not show any hematoma or abnormal bleeding. The procedure was performed quickly. After surveillance in the recovery room, the patient was taken to the surgical floor. Spleen analysis did not reveal any parenchymal abnormalities. The postoperative hospital stay was uneventful and the patient was discharged 5 days after surgery. Anticoagulation was changed to eliquis (apixaban) 15 days after discharge.
Discussion
Direct oral anticoagulants (DOACs) such as apixaban, rivaroxaban and edoxaban work by inhibiting factor Xa. The indications for the use of these DOACs have expanded significantly in recent years. They are used for primary prevention after surgical procedures with a risk of deep vein thrombosis, for the treatment of deep vein thrombosis and pulmonary embolism, and for long-term use in patients with non-valvular atrial fibrillation [1]. Clinicians generally prefer their use to that of anti-vitamin K drugs because of their rapid onset of action, fewer drug and food interactions, and the absence of continuous coagulation monitoring [2]. But bleeding remains the main side effect of this drug. It affects 6.8% to 28% of patients, and most often takes the form of mild, non-threatening mucosal bleeding. However, 3.6% of patients will experience major bleeding [2]. Two-thirds of the total ingested dose of rivaroxaban is metabolically degraded via CYP3A4 and CYP2J2 and equitably eliminated via the renal and fecal routes. The remaining third is excreted directly in the kidney. Rivaroxaban is also a substrate of the P-glycoprotein (P-gp) [1]. Many drugs are substrates, inhibitors or inducers of CYP3A4 and P-gp. Without being exhaustive, we can mention antifungals, antiretrovirals, macrolides and amiodarone as inhibitors of the CYP3A4 enzyme but also of P-gp. Thus, the concomitant use of drugs that inhibit CYP3A4 or P-gp, as well as impaired renal function, are elements that favor the risk of bleeding under direct oral anticoagulant therapy [1]. Of the 6 cases of splenic rupture we found in literature [3-8], only one reported the concomitant use of amiodarone and rivaroxaban [4]. In our case, this combination can be theoretically retained as a factor favoring spontaneous splenic rupture. The cause of the splenic rupture could therefore be totally spontaneous, considered as a side effect of rivaroxaban but also as the consequence of a drug interaction favored by an alteration in renal function [9]. However, the drug interaction between these two drugs is reported as weak in the literature [1] and splenic rupture is not described as a side effect of rivaroxaban [2]. These results from which recommendations are derived come from large databases. These databases are first compiled from trials carried out before the drug is marketed [2] and then supplemented with information from the follow-up of patients [1]. It is therefore important to report every event that may be related to the drug, as this can produce a more accurate database for the future. In the reported cases, there was a rather short delay, ranging from 1 day to 2 months, between the initiation of the anticoagulant and the atraumatic rupture of the spleen. The appearance of acute pain in the left hypochondrium, a decrease in hemoglobin and arterial hypotension are signs of acute hemorrhage. An emergency CT scan can help direct the diagnosis, define the grade of severity of the splenic injury, and thus aid in the therapeutic decision. Primary management includes discontinuation of anticoagulants, blood transfusion and crystalloid infusion [10]. Conservative treatment with close monitoring in the intensive care unit is attempted if the patient is stable and non-peritoneal. Selective arterial embolization may also be an option for stable patients. For unstable or peritoneal patients, emergency laparotomy splenectomy is required. It should be noted that "antidotes" such as andexanet alfa, a specific reversal agent for factor Xa inhibitors, have appeared on the market and could be used in situations of uncontrolled, life-threatening bleeding [10]. However, its use has not been yet reported in cases of spontaneous splenic rupture. Other measures, such as the administration of prothrombin complex concentrate or recombinant factor VIIa should be considered in cases of uncontrolled bleeding [10]. There is no data on the use of tranexamic acid. Dialysis is also not a good option because rivaroxaban is highly bound to plasma proteins. In the case of surgical management, post-operative monitoring is standard. If the patient is adequately resuscitated and managed in a timely manner, the hospital stay does not exceed one week. After hospitalization, adequate patient education is necessary to prevent infections, especially with encapsulated germs. It is recommended to be vaccinated at least two weeks after splenectomy with the pneumococcal vaccine, the ACWY meningococcal vaccine and also the group B meningococcal vaccine. If no drug interactions can be identified, rivaroxaban or another direct anticoagulant drug can be restarted remotely after the procedure [1]. Especially when the main cause of bleeding is eliminated and treated (Table 1 & 2) (Figure 1).
References
- Steffet J, Collins R, Matthias A, Cornu P, Desteghe L, et al. (2021) EHRA Practical guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Europace 23(10): 1612-1676.
- Xarelto (2021) Amsterdam: European medicines agency; c2008-2021. Xarelto: EPAR – Product information.
- Hernandez A, Ronquete M, Perez R, Vilas F (2019) Spontaneous splenic rupture of a patient treated with rivaroxaban. Clin Case Rep Int 3(1115): 1-2.
- Nagaraja V, Cranney G, Kushwaha V (2018) Spontaneous splenic rupture due to rivaroxaban. BMJ Case Rep 2018: bcr2017223237.
- Amin A, Safaya A, Ronny F, Islam H, Bhuta K, et al. (2016) Hemorrhagic shock from spontaneous splenic rupture requiring open splenectomy in a patient taking rivaroxaban. Am Surg 82(2): E54-E55.
- Naseem Z, Mustaev M, Strekozov B (2016) Spontaneous splenic rupture secondary to rivaroxaban: rare but raising. Int J Surg Med 2(3): 134-136.
- Yousef H, Daniel S, Omar B (2015) Rivaroxaban causing spontaneous splenic rupture. Am J Respir Crit Care Med 191: A4632.
- Gonzva J, Patricelli R, Lignac D (2014) Spontaneous splenic rupture in a patient treated with rivaroxaban. Am J Emerg Med 32(8): 950.
- Renzulli P, Hostettler A, Schoepfer A, et al. (2009) Systematic review of atraumatic splenic rupture. Br J Surg 96(10): 1114-1121.
- Haas S, Bode C, Norrving B, et al. (2014) Practical guidance for using rivaroxaban in patients with atrial fibrillation: balancing benefit and risk. Vasc Health Risk Manag 10: 101-114.






























