Early Radiographic and Clinical Outcomes of Three Patients Undergoing Open Instrumented Posterolateral Fusion Surgery with MagnetOsTM Easypack Putty
Christopher Elia*, DO, Megan Brais, RN, Casey Butrico, PhD and Katherine Sage, MS, DO
Submission: March 28, 2024; Published: April 16, 2024
*Corresponding author: Christopher Elia, DO 30701 Woodward Ave, Suite 301 Royal Oak, MI 48073, USA
How to cite this article: Christopher E, Megan Brais, R, Casey B, Katherine S. Early Radiographic and Clinical Outcomes of Three Patients Undergoing Open Instrumented Posterolateral Fusion Surgery with MagnetOsTM Easypack Putty. JOJ Case Stud. 2024; 14(4): 555895. DOI: 10.19080/JOJCS.2024.14.555895.
Abstract
An important factor influencing spinal fusion outcomes is the choice of bone graft. Autograft is considered the gold standard bone grafting material. However, harvesting quality autologous bone from the iliac crest may require an additional procedure and can lead to complications such as extraction site pain. Next-generation calcium phosphate bone grafts provide surgeons an option without added cells, growth factors, or the need for autologous bone. MagnetOs is a biphasic calcium phosphate bone graft with a unique needle-shaped submicron surface topography. The surface of MagnetOs predictably polarizes M2 macrophages, which in turn secrete chemokines that stimulate mesenchymal stem cell (MSC) recruitment. Signaling from M2 macrophage cytokines stimulate MSCs to differentiate into osteoblasts, which lay down osteoid for healthy bone formation. To optimize the handling characteristics of MagnetOs biphasic calcium phosphate granules, MagnetOs Easypack Putty was developed containing a polyethylene glycol-polylactic acid (PEG-PLA) polymer. This study includes four clinical cases where MagnetOs Easypack Putty was used on-label in the posterolateral spine. Radiographic fusion and patient reported outcomes demonstrate favorable outcomes using MagnetOs Easypack Putty.
Keywords: Posterolateral fusion; MagnetOs easypack putty; Osteoimmunology; Bone graft; M2 Macrophage
Introduction
The annual number of spinal fusion surgeries continues to rise in the United States, but the pseudoarthrosis rate remains the same [1,2]. Evaluating choice of bone graft can significantly improve fusion rates during spinal surgery [1,3,4]. Autologous bone, or autograft, remains the gold standard grafting material. However, an additional procedure is often necessary to obtain quality iliac crest bone graft, and local bone is limited based on procedure [5]. Furthermore, the quality of autograft varies based on the patient’s biological factors such as age and comorbidities [6,7]. Other categories of bone grafts were developed to overcome the drawbacks of using autograft. Cell based allografts, demineralized bone matrices, and allograft each require a human donor and processing to remove biological contaminants. Other graft technology involves added peptides or growth factors. However, clinical studies with peptide-based grafts have reported migration from the site of the fusion, and grafts with growth factors can lead to ectopic bone formation [8,9]. Next-generation synthetic grafts were developed to reduce or eliminate the need for autograft while mitigating the drawbacks of other advanced bone grafting devices.
MagnetOs is a biphasic calcium phosphate bone graft that contains a unique submicron needle-shaped surface topography (NeedleGripTM). The NeedleGrip technology of MagnetOs facilitates traction of pro-healing M2 macrophages [10,11*] Macrophages are monocyte-derived cells that polarize according to environmental cues. M1 macrophages are pro-inflammatory, secreting cytokines that promote fibroblast proliferation, leading to scar tissue formation [12]. M2 macrophages secrete pro-healing cytokines and chemokines that recruit mesenchymal stem cells (MSCs) to the site of MagnetOs [10,11,13*]. In response to M2 macrophage cytokines, MSCs differentiate into osteoblasts. Osteoblasts are responsible for bone mineralization through the production of enzymes such as osteonectin and alkaline phosphatase [11*]. MagnetOs Granules predictably polarize M2 macrophages for a pro-healing response that promotes healthy osteoid formation [10,13,14*].
With respect to handling properties, MagnetOs Easypack Putty contains the technology of MagnetOs Granules combined with a polyethylene glycol-polylactic acid (PEG-PLA) polymer. The PEG-PLA polymer is water soluble and biocompatible, resorbing within three days [15]. The polymer makes up 5% of the volume of MagnetOs Easypack Putty, creating a moldable graft while retaining the mechanism of action of the MagnetOs Granules [15].
This paper provides a case series of three patients who received MagnetOs Easypack Putty on-label, mixed with local morselized autograft, in open posterolateral fusion (PLF). Patients included males and females ages 46-74 with diverse medical and surgical histories. These cases report efficacy of MagnetOs Easypack Putty as measured by functional outcomes, pain scores, and radiographic success.
Case 1
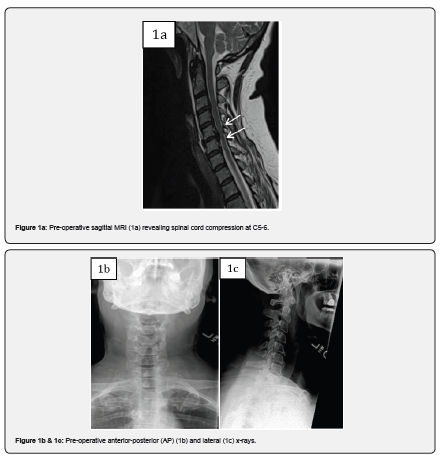
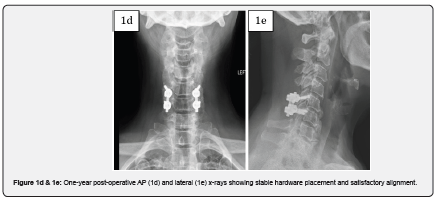
Case 1 is a 59-year-old female with complaints of progressive bilateral upper extremity radiculopathy, loss of balance and loss of dexterity. She was diagnosed with cervical spondylitic myelopathy with radiculopathy. On presentation, the patient had an Oswestry Disability Index (ODI) score of 66. Past surgical history included appendectomy and tonsillectomy. Past medical history included depression, hypertension, chronic kidney disease, low back pain and migraines. Magnetic resonance imaging (MRI) revealed spinal cord compression at C5-6. CT revealed ossification of the posterior longitudinal ligament (OPLL). The patient underwent a posterior C5-6 decompression with instrumentation and fusion with MagnetOs Easypack Putty mixed with autograft. The post-operative course was uneventful with improvement in myelopathic symptoms and resolution of radiculopathy. A post operative ODI score of 38 was recorded. Post-operative x-rays 1 year after surgery show stable hardware placement with no evidence of complication.
Case 2
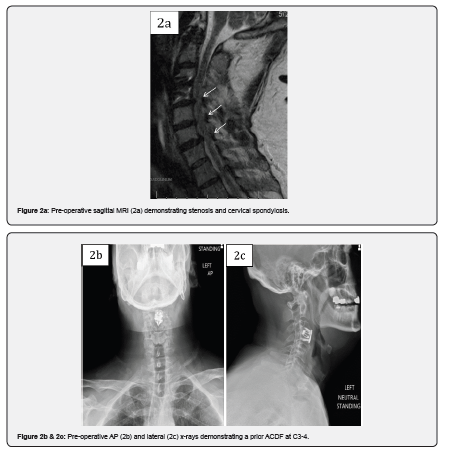
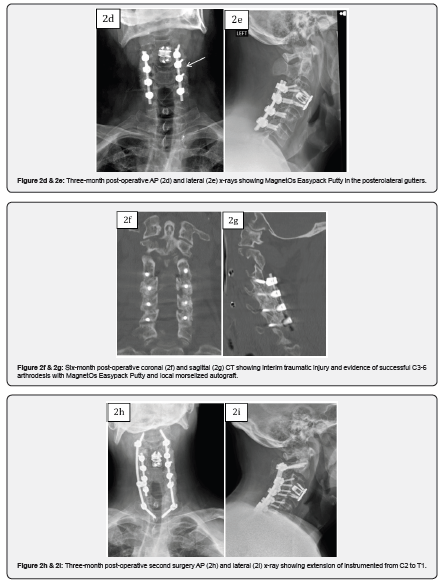
Case 2 is a 46-year-old male who presented with symptoms of worsening cervical spondylitic myelopathy manifesting as loss of dexterity and loss of balance. Past surgical history included prior C3-4 anterior cervical discectomy and fusion (ACDF) for treatment of spinal cord injury with recovery of nearly all function. Six months post-operatively the patient noted worsening myelopathic symptoms. ODI upon presentation was 68. The patient had an MRI cervical spine and was found to have multiple levels of cervical spondylosis with spinal cord compression. He underwent a posterior C3-6 decompression and fusion using MagnetOs Easypack Putty mixed with local morselized autograft. The patient demonstrated improvement in myelopathic symptoms after surgery and his 3-month ODI score improved to 42. Six months post-operative, the patient was involved in a motor vehicle accident and sustained a bilateral fracture of C2 and a laminar fracture of C7. CT performed at the time of admission revealed evidence of fusion across the facet joints of C3-6. The patient later underwent posterior C2-T1 instrumentation and fusion and was eventually discharged to rehabilitation.
Case 3
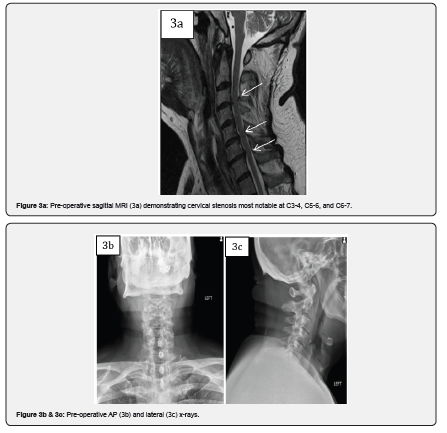
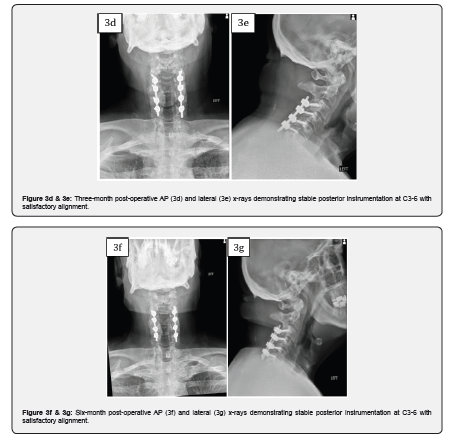
Case 3 is a 74-year-old male who presented with left upper extremity radiculopathy and cervical spondylitic myelopathy manifesting as loss of balance and loss of dexterity. Past medical history was significant for carotid endarterectomy, chronic obstructive pulmonary disease, and emphysema. ODI upon presentation was 16. MRI cervical spine revealed spinal cord compression most notable at C3-4, C5-6 and C6-7. The patient underwent a posterior cervical laminectomy at C3-4, C4-5, C5-6, and C6-7 (partial) with C3-6 instrumented fusion with 10cc of MagnetOs Easypack Putty and local morselized autograft. The patient had improvement in his left upper extremity radiculopathy, and his 6-month ODI score improved to 2.
Discussion
Time to fusion and patient reported outcomes are two measures of the success of spinal fusion surgeries. However, radiographic outcomes can be analyzed with a variety of imaging modalities and fusion criteria, with inconsistencies in literature [3]. Utilizing multiple imaging modalities at various time points provides comprehensive observations to evaluate time to fusion and extent of bone formation.
This case series of patients underwent posterolateral instrumented arthrodesis with MagnetOs Easypack Putty with satisfactory clinical outcomes. MagnetOs Easypack Putty was used on-label as an extender in the posterolateral spine in the cervical spine. Post-operative imaging includes three-month, six-month, and/or one-year follow-up. Case 2 demonstrated radiographic signs of successful arthrodesis on CT scan 6 months after the index operation.
MagnetOs promotes bone formation in the absence of added growth factors or human-derived cells. Bone is formed throughout the graft in response to the sub-micron needle-shaped topography, which provides traction for pro-healing M2 macrophages [10,11*]. In response, MagnetOs forms bone during PLF through core and creeping edge repair [14*]. The PEG-PLA polymer in MagnetOs Easypack Putty was optimized to maintain the surface topography of MagnetOs Granules while creating a malleable, ready-to-use graft formulation composed of 87% PEG and 13% PLA [15]. Pre-clinical radiographic and histologic analyses reveal resorption of the PEG-PLA polymer within three days and resorption of the biphasic calcium phosphate granules accompanied by mature bone formation within three months [15*]. No graft migration or adverse events were reported [15]. Although we do not have 1 year post-operative CT on all 3 patients, one of the patients demonstrated evidence of spinal fusion on postoperative CT scan 6 months after surgery. This publication provides a clinical case series that demonstrates safe clinical use of MagnetOs Easypack Putty and satisfactory post-operative outcomes in the three patients undergoing posterolateral fusion of the cervical spine.
Conclusion
This case series demonstrates positive patient reported outcomes of MagnetOs Easypack Putty in a retrospective cohort of patients undergoing posterolateral fusion of the cervical spine.
Conflict of Interest
The study was supported by a grant from Kuros Biosciences BV. CE is a consultant for Kuros Biosciences BV. CB and KS have financial disclosures with Kuros Biosciences BV.
*Results from in vivo laboratory testing may not be predictive of clinical experience in humans. For important safety and intended use information please visit kurosbio.com.
References
- Hsu WK, Nickoli MS, Wang JC, Lieberman JR, An HS, et al. (2012) Improving the Clinical Evidence of Bone Graft Substitute Technology in Lumbar Spine Surgery. Global Spine J 2(4): 239-248.
- Mabud T, Norden J, Veeravagu A, et al. (2016) Complications, Readmissions, and Revisions for Spine Procedures Performed by Orthopedic Surgeons Versus Neurosurgeons A Retrospective, Longitudinal Study.
- Chun DS, Baker KC, Hsu WK (2015) Lumbar pseudarthrosis: A review of current diagnosis and treatment. Neurosurg Focus 39(4): E10.
- Morris MT, Tarpada SP, Cho W (2018) Bone graft materials for posterolateral fusion made simple: a systematic review. European Spine Journal 27(8): 1856-1867.
- Dimitriou R, Mataliotakis GI, Angoules AG, Kanakaris NK, Giannoudis PV (2011) Complications following autologous bone graft harvesting from the iliac crest and using the RIA: A systematic review. Injury 42 Suppl 2: S3-S15.
- Wilson LA, Fiasconaro M, Liu J, Bekeris J, Poeran J, et al. (2020) Trends in Comorbidities and Complications Among Patients Undergoing Inpatient Spine Surgery. Spine (Philadelphia, Pa 1976). 45(18): 1299-1308.
- Whitmore RG, Stephen J, Stein SC, Campbell PG, Yadla S, et al. Patient Comorbidities and Complications After Spinal Surgery: A Societal-Based Cost Analysis. Spine (Philadelphia, Pa 1976) 37(12): 1065-1071.
- James AW, LaChaud G, Shen J, Asatrian G, Nguyen Vi, et al. (2016) A Review of the Clinical Side Effects of Bone Morphogenetic Protein-2. Tissue Eng Part B Rev 22(4): 284-297.
- Mobbs RJ, Maharaj M, Rao PJ (2014) Clinical outcomes and fusion rates following anterior lumbar interbody fusion with bone graft substitute i-FACTOR, an anorganic bone matrix/P-15 composite. J Neurosurg Spine 21(6): 867-876.
- Duan R, van Dijk LA, Barbieri D, de Groot F, Yuan H, et al. (2019) Accelerated bone formation by biphasic calcium phosphate with a novel sub-micron surface topography. Eur Cell Mater 37: 60-73.
- Van Dijk LA, De Groot F, Yuan H, Campion C, Patel A, et al. From benchtop to clinic: A translational analysis of the immune response to submicron topography and its relevance to bone healing. Eur Cell Mater 41: 756-773.
- Maxwell LJ, Zochling J, Boonen A, Singh JA, Veras MMS, et al. (2015) TNF-alpha inhibitors for ankylosing spondylitis. Cochrane Database of Systematic Reviews (4): CD005468.
- van Dijk LA, Utomo L, Yuan H, De Groot BF, Gawlitta D, et al. (2023) Calcium phosphate with submicron topography influences primary human macrophage response, enhancing downstream angiogenesis and osteogenesis in vitro. J Immunol Regen Med 19: 100070.
- van Dijk LA, Duan R, Luo X, Barbieri D, Pelletier M, et al. (2018) Biphasic calcium phosphate with submicron surface topography in an Ovine model of instrumented posterolateral spinal fusion. JOR Spine 1(4): 01039.
- Belluomo R, Arriola-Alvarez I, Kucko NW, Walsh WR, de Bruijn JD, et al. (2022) Physico-Chemical Characteristics and Posterolateral Fusion Performance of Biphasic Calcium Phosphate with Submicron Needle-Shaped Surface Topography Combined with a Novel Polymer Binder. Materials 15(4): 1346.






























