A Rare Case in the Newborn: Craniofrontonazal Syndrome
Samet Benli*, Atika Çağlar and Mustafa Aydın
Department of Child Health and Diseases & Neonatology, Fırat University, Turkey
Submission: April 01, 2023; Published: April 12, 2023
*Corresponding author: Samet Benli, Neonatology Specialist, Fırat University Faculty of Medicine, Department of Neonatology, Elazig, Turkey
How to cite this article: Samet B, Atika Ç, Mustafa A. A Rare Case in the Newborn: Craniofrontonazal Syndrome. JOJ Case Stud. 2023; 14(2): 555881. DOI: 10.19080/JOJCS.2023.14.555881.
Abstract
Craniofrontonasal syndrome (CFNS) is a very rare X-linked inherited disease characterized by abnormalities in the head and face, hands and feet, and certain skeletal bones, first described by Professor Michael Cohen from Canada in 1979. The phenotypic trait varies greatly among affected individuals; The syndrome is more common in girls and more severely affected than boys. The incidence of the disease is rare.
Keywords: Newborn; Craniofrontonasal syndrome; Craniosynostosis
Introduction
Craniofrontonasal syndrome (CFNS) (OMIM #304110) was first described by Cohen in 1979 and is an X-linked disease caused by mutations in the Ephrin B1 gene (EFNB1) located on the short arm of the X chromosome (Xp13.1) [1,2]. Affected girls present with the clinical picture, coronal craniosynostosis (CS), frontal prominence, hypertelorism, depressed nasal bridge, bipolar nose, craniofacial asymmetry, downward-sloping palpebral fissures, curly and curly hair, syndactyly, and longitudinal striation on the nails. Interestingly, many symptomatic hemizygous men show only hypertelorism with no other congenital anomalies or major facial dysmorphism [3,4]. It is also possible that craniofrontonasal syndrome may be a gender-limited disorder, which is more severe in girls, explained by the differential interaction of the mutant gene with sex-specific developmental pathways [5]. We wanted to discuss the case, which was diagnosed as craniofrontonasal syndrome, in order to be seen as rare and to be educational, in the light of the literature.
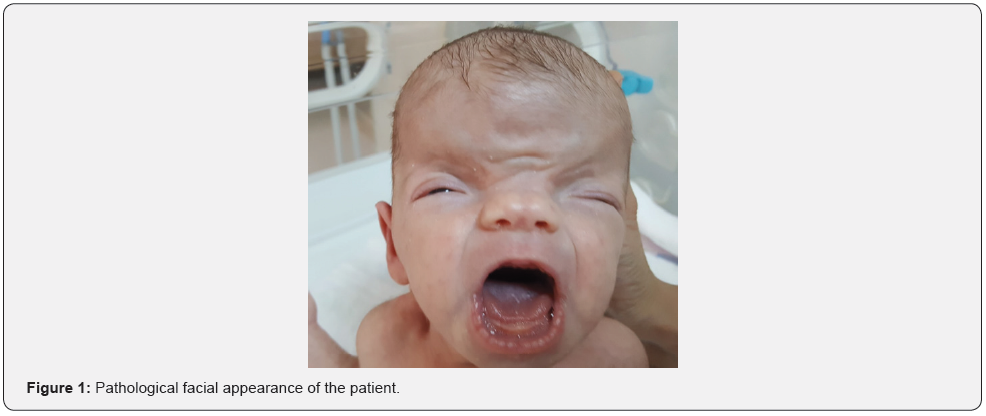
Case Report
A female baby born by cesarean section from a 28-yearold mother was admitted to the neonatal intensive care unit for diagnosis and treatment because of its syndromic appearance. The other two siblings of the patient, whose pregnancy follow-ups were not regular and there was no feature in her family history, were alive and healthy. In the first examination of the patient, body weight was measured as 2700g (25-50 p), height 47cm (25- 50 p), head circumference (10-25 p). The patient had pathological physical examination findings of brachiocephaly, frontal bossing, hypertelorism, slightly downward sloping palpebral fissures, wide nasal bridge, bifid nasal tip (Figure 1). The neck was short and low ears were present. Nipples were wide and asymmetrically located. The patient’s entire abdomen and cranial ultrasonography and echocardiography were normal. Craniosynostosis was detected in the patient’s brain magnetic resonance imaging. Karyotype analysis was sent from the patient and it was normal as 46,XX. A mutation in the EFNB1 gene was found in the patient who was thought to have Craniofrontonasal syndrome based on clinical and radiological findings. The patient was discharged from the neonatal intensive care unit after 21 days postnatally with recommendations.
Discussion
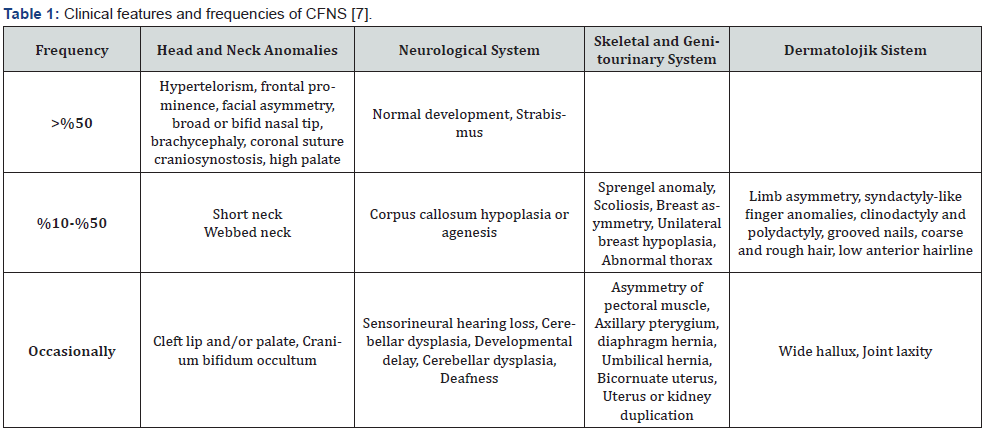
The classic definition of CFNS includes facial asymmetry due to coronal craniosynostosis, hypertelorism, broad or bifid nasal tip, and brachycephaly. However, clinical manifestations are highly variable even within the same family. Except for craniofacial malformation, extracranial features are not uncommon [6]. The clinical findings of CFNDS are summarized in Table 1 [7].
The interesting “genetic paradox” associated with CFNS can be explained by the specific features of the EFNB1 gene. The EFNB1 gene was identified as the gene that causes CFNS in 2003 and is located on the X chromosome in the Xq13.1 region. The EFNB1 gene encodes the transmembrane protein ephrin-B1, a ligand for ELK. Its main function is to control cell sorting, migration and growth, which are crucial for tissue morphogenesis [8]. In hemizygous males, due to random binding, the ephrin-B1 deficit may be compensated for by other ephrin molecules, resulting in a less severe clinical phenotype. However, in heterozygous girls, cell-cell interactions are more complicated in the presence of random X inactivation. With X inactivation, a mosaic pattern of broad-type and mutant ephrin-B1 proteins is present. The mutant protein will interfere with the broad type, a phenomenon known as cellular interference, and explains the “genetic paradox” seen in CFNS. Different X inactivation patterns may also explain the variation in expression within the family [7,8].
CFNS is an X-linked syndrome because females are affected while male carriers are not affected at all or show very mild abnormalities [1,2]. Often, the condition is not diagnosed in boys unless they are a member of a family known to have the disease or father of a girl with the disease. An affected woman has a 50% chance of her daughter or son getting the disease. An affected male will have the disease transmitted to all of his daughters, but not to any of the males [2,3]. In the differential diagnosis, (MIM 145420) is similar to the findings in craniofrontonasal syndrome such as brachycephaly, telecanthus/hypertelorism and wide nasal tip. However, the absence of craniosynostosis and brittle linear nails are the main points that distinguish the disorder from craniofrontonasal syndrome [9].
Management of CFNS is similar to that of other craniosynostotic syndromes and requires a multidisciplinary team approach. However, CFNS is more challenging on surgical technique due to variable craniosynostosis, craniofacial asymmetry and hypertelorism. Craniosynostotic correction has been recommended for 3 to 6 months to prevent increased intracranial pressure. Facial bipartition for facial asymmetry and hypertelorism should be performed at approximately 5-6 years of age after eruption of the maxillary central teeth to prevent severe disruption of the occlusal plane. Correction of nasal deformity should also be done during the facial bipartition period and should be revised in the adolescence period when the skeletal system matures [10]. Since strabismus and dissociated eye movements are more common in CFNS, regular ophthalmologic examination is recommended for early detection of visual impairment and timely intervention [11].
Conclusion and Recommendation
Genetic counseling and a multidisciplinary approach can be beneficial for affected individuals and their families. Each new case will serve as a guide to help clarify the phenotype of craniofrontonasal syndrome in the future.
References
- Cohen MM Jr (1979) Craniofrontonasal dysplasia. Birth Defects Orig Artic Ser 15(5B): 85-89.
- Wieland I, Jakubiczka S, Muschke P, Cohen M, Thiele H, et al. (2004) Mutations of the ephrin-B1 gene cause cranio-frontonasal syndrome. Am J Hum Genet 74(6): 1209-1215.
- Twigg SRF, Babbs C, van den Elzen MEP, Goriely A, Taylor S, et al. (2013) Cellular interference in craniofrontonasal syndrome: males mosaic for mutations in the X-linked EFNB1 gene are more severely afected than true hemizygotes. Hum Mol Genet 22(8): 1654-1662.
- Van Den Elzen MEP, Twigg SRF, Goos JAC, Hoogeboom AJM, Van Den Ouweland AMW, et al. (2014) Phenotypes of craniofrontonasal syndrome in patients with a pathogenic mutation in EFNB1. Eur J Hum Genet 22(8): 995-1001.
- Wieland I, Makarov R, Reardon W, Tinschert S, Goldenberg A, et al. (2008) Dissecting the molecular mechanisms in craniofrontonasal syndrome: differential mRNA expression of mutant EFNB1 and the cellular mosaic. Eur J Hum Genet 16(2): 184-191.
- Luk HM, Lo IFM, Tong TMF, Lam STS (2015) Craniofrontonasal Dysplasia: A Report of Two Chinese Families and Literature Review. HK J Paediatr (new series) 20: 105-109.
- Wieacker P, Wieland I (2005) Clinical and genetic aspects of craniofrontonasal syndrome: towards resolving a genetic paradox. Mol Genet Metab 86(1-2): 110-116.
- Murai KK, Pasquale EB (2003) "Eph"ective signaling: forward, reverse and crosstalk. J Cell Science 116(Pt 14): 2823-2832.
- Baltimore, MD, Johns Hopkins University, ONLINE MENDELIAN INHERITENCE IN MAN (OMIM). Craniofrontonasal dysplasia (MIM #304110), Teebi hypertelorism syndrome (MIM 145420), Craniofacial Dyssynostosis syndrome (MIM 218350).
- Kawamoto HK, Heller JB, Hellet MM, et al. (2007) Craniofrontonasal dysplasia: a surgical treatment algorithm. Plast Reconstr Surg 120(7): 1943-1956.
- Tay T, Martin F, Rowe N, et al. (2007) Visual manifestations of craniofrontonasal dysplasia. J Pediatr Ophthalmol Strabismus 44(4): 251-254.






























