Sequelae of Partial Treatment in Congenital Adrenal Hyperplasia: A Case Report
Jovaria Ehsan*, Shahla Zameer, M Ehsan ul Haq and Saima Shan
Associate Radiologist, Federal Government Polyclinic Hospital, Pakistan
Submission: January 18, 2023; Published: February 09, 2023
*Corresponding author: Jovaria Ehsan, Associate Radiologist, Federal Government Polyclinic Hospital, Islamabad, Pakistan
How to cite this article: Jovaria Ehsan, Shahla Zameer, M Ehsan ul Haq and Saima Shan. Sequelae of Partial Treatment in Congenital Adrenal Hyperplasia: A Case Report. JOJ Case Stud. 2023; 14(1): 555879. DOI: 10.19080/JOJCS.2023.14.555879.
Abstract
Congenital adrenal hyperplasia is a rare genetic mutational disease in enzymes related to steroidogenesis. Majority of the patients are virilized and have stunted growth and development. Goal of treating such patients is to maintain an adequate hormonal profile balanced with suppression of ACTH. Failure to do so may result in bulky adrenal glands, accelerated skeletal maturation and precocious puberty. We present an unusual, partially treated case of congenital adrenal hyperplasia with gross and symmetrical enlargement of bilateral adrenal glands and multicystic ovaries on various imaging modalities.
Keywords: Congenital adrenal hyperplasia; Steroid therapy; Precocious puberty
Introduction
Congenital adrenal hyperplasia is a rare autosomal recessive condition resulting due to enzymatic deficiency related to steroidogenesis [1]. With an incidence of 1 in 10,000 live births worldwide [1,2], more than 95 percent of cases are due to deficiency of 21 alpha hydroxylase, less being deficiency of 17 alpha hydroxylase and 11 beta hydroxylase [2]. Three phenotypic forms based on these deficiencies are categorized as classical salt wasting, classical simple virilizing and late onset non classical disease pattern [3]. Infants suffering from congenital adrenal hyperplasia are either born with ambiguous genitalia [4] or suffer from salt wasting [3].
Aim of therapy in such patients is to synchronously correct the deficiency of cortisol and to suppress overproduction of ACTH [5]. Proper glucocorticoid therapy suppresses the androgen steroidogenesis and
prevent virilization and allow normal growth and development of the child [4]. Failure to achieve this may result in enlargement of adrenal glands along with accelerated skeletal maturation manifesting as short stature and various genital abnormalities [1,4,5]. Adequate dosing of steroids is vital in infancy and childhood so that adequate growth and development is achieved [6]. We present a unique case of a 10 year old diagnosed case of congenital adrenal hyperplasia whose dose of corticosteroids was not adjusted according to her age, causing short stature, massive adrenal enlargement and bilateral ovarian cysts.
Case Report
A ten years old girl, eldest child in sibship of three, came in Radiology department for an abdominal ultrasound with complaints of lower abdominal pain. She had menarche at age of 9 years, her periods being irregular and scanty. She was short statured as compared to her age and had excessive facial hair. Upon further inquiry her mother said that she was born with ambiguous genitalia and hyper pigmentation, for which she was prescribed various tests for the confirmation of diagnosis of congenital adrenal hyperplasia. Financial and social constraints of parents along with non-compliance caused a delay in her diagnosis till the child was 1 year of age, after which her treatment ensued. She was prescribed a morning dose of a quarter of a tablet of 10mg of hydrocortisone and 1 tablet of fludrocortisone 0.1mg daily. At age of 9 years, she underwent clitoral reduction. The parents failed to maintain a regular follow-up however continued the child on earlier prescribed dosage for about 10 years. Despite of treatment she didn’t achieve an adequate height and started menstruation at a relatively early age of 9 years associated with frequent episodes of lower abdominal pain with hirsutism. Her radiological bone age was of 13 years old girl showing accelerated skeletal maturation.
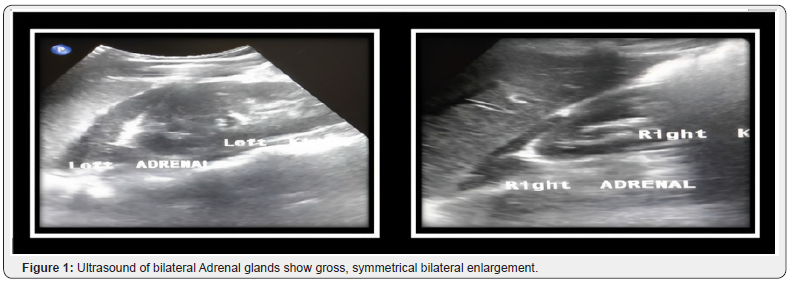
Her ultrasound showed bilateral symmetrically enlarged adrenal glands with characteristic cerebriform pattern. Both limbs of adrenal gland were grossly enlarged measuring over 4cm (Figure 1).
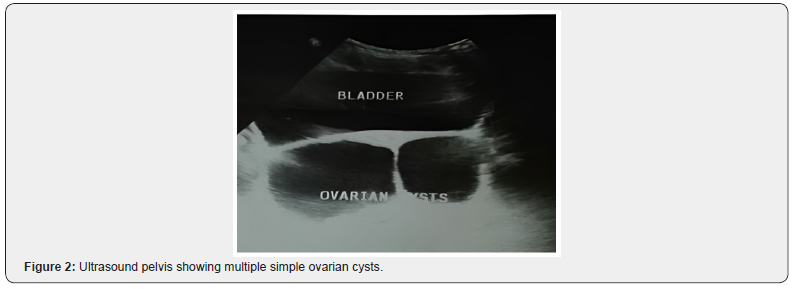
Ultrasound of pelvis showed at least three simple follicular cysts in right ovary and four cysts in left ovary. Uterus however was unremarkable (Figure 2).
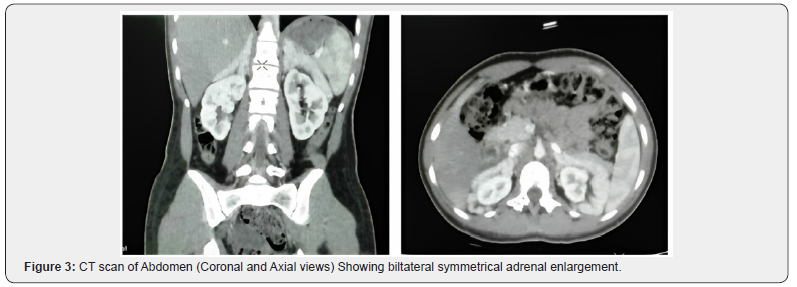
As she was a known case of congenital adrenal hyperplasia , her CT scan abdomen with adrenal protocol was also done to rule out myelolipomas in adrenal glands as her adrenal glands were grossly enlarged. Her CT scan showed grossly enlarged bilateral adrenal glands which were symmetrical and showed no focal enhancing lesion (Figure 3).
She was referred back to Pediatrics department for regulation and review of the dosage. She was prescribed half a tablet of 5mg deltacortil twice daily and 1 tablet of 0.5mg fludrocortisone. She was followed for a period of six months whereby her complaints of lower abdominal pain settled with regularization of her menses with normal serum cortisol levels. Follow up ultrasound showed a normal ovarian follicular pattern.
Discussion
Congenital adrenal hyperplasia constitutes a group of autosomal recessive enzymatic deficiencies related to the adrenal steroidogenic pathway resulting in a spectrum of endocrine and metabolic abnormalities [1]. Based upon enzymatic activity, it is divided into three phenotypic forms, classical salt wasting, classical simple virilizing and late onset non classical disease pattern [3]. Our patient was diagnosed as a case of classical simple virilizing variety based upon her infantile history, clinical examination and hormonal assay.
More than 90 percent of cases are due to deficiency of 21 alpha hydroxylase which catalyzes conversion of 17-hydroxyprogesterone to 11-deoxycortisol and progesterone to deoxycorticosterone, resulting in their accumulation and shunting of these precursors to excess androgen production [5]. Increased androgens cause ambiguous genitalia, hirsutism and later precocious puberty [3,7]. Our patient too had acquired puberty at an early age of 9 years with ambiguous genitalia at birth.
Excess androgens and inadequate steroid therapy during childhood results in rapid growth with early fusion of epiphysis, affecting final height [3]. Radiological bone age assessment of our patient also showed early fusion of epiphyses corresponding to bone age of 13 years at the chronological age of 10 years.
Due to impaired cortisol production associated with absence of negative feedback mechanism of adrenocorticotropic hormone, its excess may lead to hyperplasia of bilateral adrenal glands [1,2,8] however not as profound as our patient had with demonstration of characteristic cerebriform pattern on ultrasound and homogenous symmetrical enlargement on contrast enhanced computed tomography.
Excessive circulating androgens also exert influence on hypothalamus and anterior pituitary axis disrupting LH/FSH ratio and affecting GnHRH, causing arrested maturation of ovarian follicles leading to multiple cyst formation [9-11]. In consistence to the studies done worldwide, our patient also had multiple bilateral ovarian cysts.
Treatment of such children with adequate dosing according to transition of age is of key importance so that adequate skeletal maturation be achieved with alleviation of genital problems [6,7]. Our patient was continued on the infantile dose which was prescribed to her earlier without increments or appropriate dosing.
Once our patient’s steroid dosing was prescribed, her cortisol levels became under control and her problems slowly started to abate.
Conclusion and Recommendation
Main aim of therapy is thus targeted to cohesively reduce excess androgens, suppress ACTH, and ensure effective replacement of glucocorticoids and mineralocorticoid for longevity and quality of life of the patient. Early diagnosis, prompt ensue of treatment and follow up is the key during transition from childhood to adulthood.
References
- Kok HK, Sherlock M, Healy NA, Doody O, Govender P, et al. (2015) Imaging features of poorly controlled congenital adrenal hyperplasia in adults. The British Journal of Radiology 88(1053): 20150352.
- Teixeira SR, Elias PC, Andrade MT, Melo AF, Elias Junior J (2014) The role of imaging in congenital adrenal hyperplasia. Arquivos Brasileiros de Endocrinologia & Metabologia 58: 701-708.
- El-Maouche D, Arlt W, Merke DP (2017) Congenital adrenal hyperplasia. The Lancet 390(10108): 2194-2210.
- Byne W (2006) Developmental endocrine influences on gender identity. The Mount Sinai Journal of Medicine 73(7):950-959.
- Speiser PW, Arlt W, Auchus RJ, Baskin LS, Conway GS, et al. (2018) Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: an Endocrine Society clinical practice guideline. The Journal of Clinical Endocrinology & Metabolism 103(11): 4043-4088.
- Hoyer-Kuhn H, Huebner A, Richter-Unruh A, Bettendorf M, Rohrer T, et al. (2021) Hydrocortisone dosing in children with classic congenital adrenal hyperplasia: results of the German/Austrian registry. Endocrine Connections 10(5): 561-569.
- Lteif AN, Zimmerman D (1996) Precocious puberty in congenital adrenal hyperplasia. In: Pediatric Research 1996 Apr 1. 351 West Camden ST, Baltimore, MD 21201-2436: Williams & Wilkins, 39(4): 537-537.
- Falke TH, van Seters AP, Schaberg A, Moolenaar AJ (1986) Computed tomography in untreated adults with virilising congenital adrenal cortical hyperplasia. Clinical Radiology 37(2): 155-160.
- Gomes LG, Bachega TA, Mendonca BB (2019) Classic congenital adrenal hyperplasia and its impact on reproduction. Fertility and Sterility 111(1): 7-12.
- Shankar R, Mahajan JK, Khanna S, Rao KL (2010) Bilateral ovarian cysts in a neonate with salt-wasting congenital adrenal hyperplasia. Journal of Pediatric Surgery 45(5): e19-e21.
- Shima M, Tanae A, Miki K, Katsumata N, Matsumoto S, et al. (2000) Mechanism for the development of ovarian cysts in patients with congenital lipoid adrenal hyperplasia. European Journal of Endocrinology 142(3): 274-279.






























