Otolaryngologic Manifestations of Wolf Hirschhorn Syndrome - A Case Report
Essa Tawfeeq, Mariam Sarkhouh* and Mutlaq Al-sihan
Department of ENT Head and Neck Surgery, Zain Hospital, Kuwait
Submission: March 18, 2022; Published: April 12, 2022
*Corresponding author: Mariam Sarkhouh, Department of ENT Head and Neck Surgery, Zain Hospital, Alsabah Medical area, Kuwait
How to cite this article: Essa T, Mariam S, Mutlaq Al-Sihan. Otolaryngologic Manifestations of Wolf Hirschhorn Syndrome - A Case Report. JOJ Case Stud. 2022; 13(3): 555864. DOI: 10.19080/JOJCS.2022.13.555864.
Abstract
In this paper, we aim to escalate the knowledge about Wolf Hirschhorn syndrome particularly regarding the clinical symptoms, but more importantly to highlight the importance of an early otolaryngologic examination in Wolf Hirschhorn Syndrome cases. Up to now, otolaryngologic manifestations of this syndrome have aroused little interest in the scientific literature. Although current relative studies have addressed that one third of WHS patients present with cleft lip and palate [1]. These patients require a thorough examination of the ear nose and throat without further due in order to prevent hearing loss which might be the consequence of recurrent ear infections resulting from cleft lip and palate in these patients. unfortunately, most of the available information in the ENT literature come from individual case studies.
Keywords: Wolf-Hirschhorn syndrome; Otolaryngology; Genetics
Introduction
One of the rare severe multisystem genetic disorders is Wolf-Hirschhorn syndrome [2]. Patients with this syndrome have special characteristics including prominent glabella, hypertelorism, broad beaked nose and frontal bossing all together are called “Greek warrior helmet” appearance [3]. Also, other facial irregularities such as broad nasal bridge, high eyebrow archaism, micrognathia, microcephaly, epicanthal folds and proptosis can occur in patients with WHS [2,3]. In addition, clefts of lip and/or palate are observed in almost half of the cases. Moreover, Developmental abnormalities, intellectual disabilities, seizures, heart defects are also considered major problems seen in patients with WHS [2,3]. Although hearing loss is rarely discussed and described in these patients, it has been confirmed that it is seen in those patients and might be missed thus a proper audiological and otologic assessment should be performed without delay [4]. WHS cases are confirmed by chromosomal analysis in which a deletion of a fundamental region on chromosome 4p16.3 is detected by chromosomal microarray or fluorescence in situ hybridization (FISH) [2]. WHS occurs from partial 4p deletions that result in the disruption of other genes which leads to different symptoms of WHS. Regarding to the level of severity of WHS symptoms, they range from one case to another based on the genetic material missing due to the 4p deletions [2,3].
Case Presentation
This paper reports on a case of WHS, addressing the associated clinical manifestations and the ENT associated abnormalities occurring in a patient with this syndrome.
M.A is an 8 -month old female. She is the first offspring of a non-consanguineous parents. Her family history was insignificant for any congenital malformations. Investigations done in the antenatal period revealed polyhydramnios, IUGR, and increased UMA diastolic flow resistance. However, amniocentesis and qad serum screening (Quad AFP, HCG, Estrio, and inhibin) were not performed to diagnose chromosomal abnormalities during 15-20 weeks of gestation. Therefore, genetic counselling was not provided for the patient’s family. Consequently, the mother did not have the option to abort or keep the fetus.
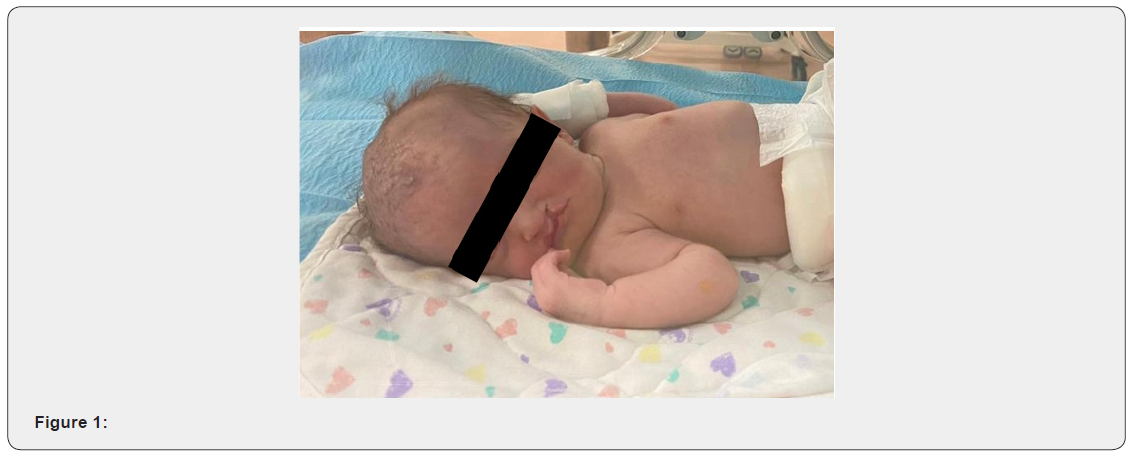
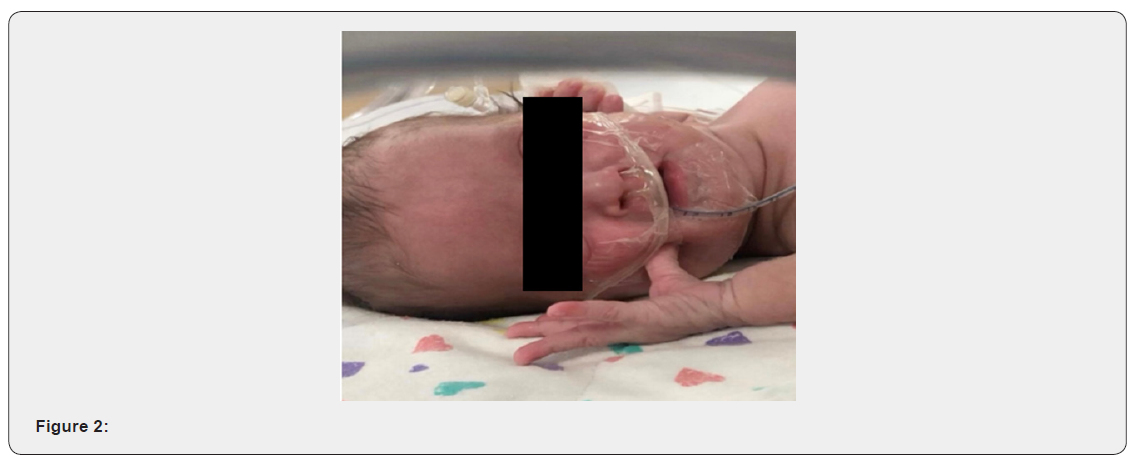
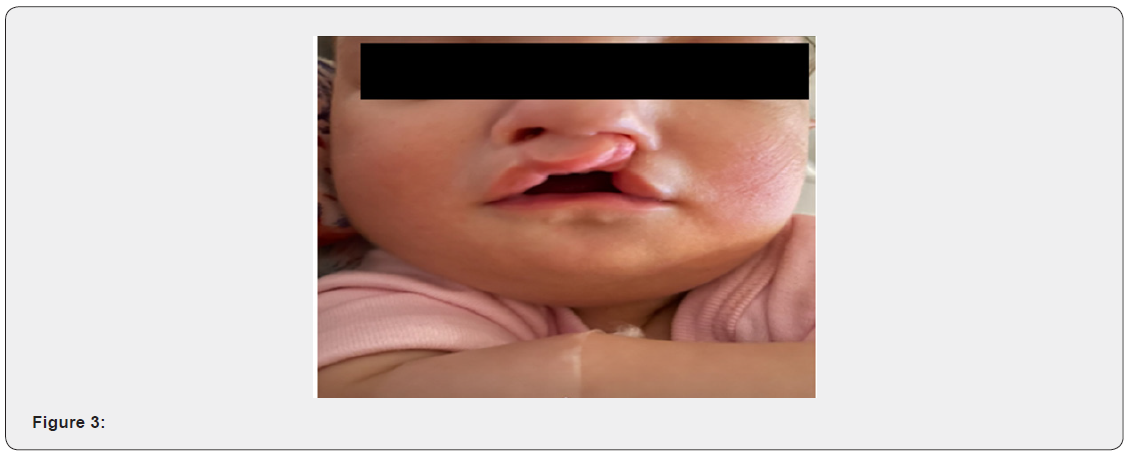
The patient was born at 37 weeks by normal vaginal delivery and was admitted to the Special care baby unit due to the dysmorphic features seen. Her birth weight was 2.155kg and length was 41cm, Apgar score was 8 and 9 respectively at one and five minutes. During her admission in SCBU2, the patient developed respiratory distress and kept on a nasal cannula then HFNC but didn’t maintain a good oxygen saturation, hence, was shifted to the NICU. During her stay in the NICU, the patient was kept on an assisted controlled ventilation for four days until shewas extubated to a nasal cannula. Furthermore, cholestasis was detected and was gradually improving with feeding. On physical examination, the patient was generally stable and her vital signs were within normal range. The craniofacial features noticed in our patient were as follow microcephaly, Greek warrior helmet appearance, wide bridge of the forehead, and high anterior hairline. Also, the patient presented with a prominent glabella, widely spaced eyes, and epicanthal folds. Moreover, right incomplete cleft lip, left cleft lip, cleft hard and soft palate, and micrognathia were also observed. Cardiovascular examination showed regular rate and rhythm and audible murmurs on auscultation. The Chest was clear with fine bilateral air entry and no respiratory distress. Her abdomen was soft with no tenderness or distention and no organomegaly. The patient underwent several Investigations such as FISH that resulted in 46, XX, del(4) (p16.1) female karyotype, confirming WHS. Additional test was Echocardiogram that showed large ASD 10mm, PDA, and probable right aortic arch. Brain MRI was also done and revealed bilateral subependymal cyst. After referring the patient to an ENT doctor, a tympanometry was done and showed B type tympanogram which is consistent with bilateral OME. A Pediatric Auditory Brainstem Response and an Auditory Steady State Response tests were also requested. The patient was found to have bilateral severe to profound mixed hearing loss in the frequency range 2 to 4 KHZ.
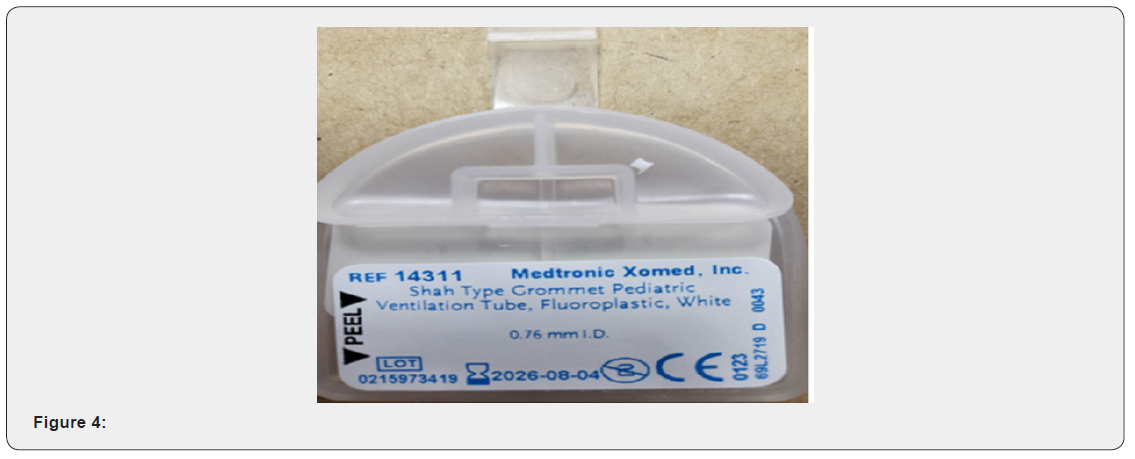
Of course, the patient’s mother was asked to follow up with a multidisciplinary team to assess and plan the management for her baby. A clef lip repair surgery was arranged on the 20th/4/2021 followed by a cleft palate repair three months later. After the cleft lip repair procedure, the patient was seen by the otolaryngologist who has decided to perform bilateral anteroinferior inferior myringotomy with bilateral tube insertion to maintain drainage. the type of those tubes were grommet pediatric ventilation tubes measured 0.76mm each. The procedure was not easy to be done as the patient’s external auditory canal measured 2mm. Therefore, the smallest cone was used to visualize tympanic membrane. Knowing that the child is having effusion and the possibility of hearing loss is high, otological investigations and interventions were mandatory. In this case an early hearing detection and intervention was done. The first test is TEOAE which is a conducted to assess how well is the inner ear and cochlea, functions. The second test was (AABR) Automated Auditory Brainstem Response in which the hearing nerve and the brain respond are both evaluated. The results were bilateral conductive and sensorineural hearing loss in the frequency range (2-4) KHZ (Figure 1-4).
Discussion
It is estimated that WHS has a frequency of approximately 1 in 20,000 to 1 in 50,000 live births, with the syndrome affecting females as twice often as males. The ratio of the cases might be miscalculated due to the inability to diagnose all those with WHS [2]. It has been shown that 35% of patients with WHS die in the first two years of life with the majority of the children survive up to 20 to 30 years. Heart defects, aspiration pneumonia, and infectious complications are considered to be the most common causes of death in these babies [2]. The genetic mechanism of Wolf-Hirschhorn syndrome is drawn on a deletion of the distal short arm (p) of chromosome 4(g, p, r). The section strip 16.3 is a critical region when it comes to WHS, therefore, 4p16.3 deletion will lead to developmental abnormalities, intellectual disability, irregular facial appearance, and so many other manifestations of the syndrome by the disruption of other genes [2-4]. Previous research has established that the severity of intellectual disability and physical abnormalities is directly associated with the size of the deletion which differs from one individual to another with the same condition [4,5]. Generally, WHS cases are caused by one of the following mechanisms, denovo deletions, familial translocation, or denovo translocations [6].
Patients with WHS mostly present with multiple distinctive features, including, facial characteristics, such as microcephaly, micrognathia and cleft lip and/or palate. Additionally, broad flat nasal bridge, high forehead, hypertelorism, and frontal bossing described as “Greek warrior helmet” appearance. Patients also have seizures, epilepsy, and mental retardation. Ocular hypertelorism, ectopia lentis, coloboma of the iris also frequently observed in those with WHS [3,6,7]. Children with this syndrome may also suffer from congenital heart defects, renal anomalies, immunodeficiency, and skeletal development retardation. Additional findings include the otolaryngology manifestations, for instance, dysplastic ears, deafness, respiratory tract infections, recurrent otitis media, and OME [1,7,8].
There are many ways to establish the diagnosis of WHS. For example, prenatal methods in which amniocentesis and fetal karyotyping are done showing partial deletion of chromosome 4(del(4p16.3)). Obstetric ultrasound also can be useful in revealing IUGR, hypoplastic kidneys, and the features suggesting “Greek warrior helmet appearance [1].
Second, postnatal methods which are very important for the assessment of WHS cases. Mainly the diagnosis of WHS is established by finding a heterozygous deletion of the critical region on chromosome 4p16.3 by conventional G-banded cytogenetic analysis, chromosomal microarray or fluorescence in situ hybridization (FISH)and comparative genomic hybridization (aCGH) [2].
However, evaluating growth parameters and plotting on a growth chart is fundamental to evaluate the extent of the disease. Physical examination for skeletal anomalies and the heart in infancy and testing for immunodeficiency is a must. Consultation with a medical geneticist and/or genetic counselor is a key step. Moreover, Comprehensive evaluation by an otolaryngologist and comprehensive audiological screening (brain stem auditory evoked responses) as early as possible should be done because language delay and educational development will be affected by hearing impairment in these patients [1,8].
Even though, otologic manifestations are commonly observed in those patients, very few studies have discussed this issue [9]. It has been reported that WHS can result in frequent serous otitis media, bilateral chronic otitis media, ossicular chain defects which can give rise to conductive hearing loss. Cochlear neuroepithelial lesions will contribute to sensorineural hearing loss in WHS [8]. Deletion of both WHSC1 and FGFR3 has been proven in previous studies to be responsible for different phenotype in patients with WHS with WHSC1 haploinsufficiency is the one responsible for hearing loss in WHS [3]. In a study done to emphasize on identifying the abnormalities that might cause hearing loss in WHS patients, it has been observed that the malleus was malformed, the passage of the chorda tympani nerve bilaterally through the bone of the malleus. In the organ of corti there was an outer hair cell loss. Additionally, defects in the bone of the apical osseous spiral lamina in both cochlea, absence of the stapes and oval window, and malformation of the incus were noted [8]. Also, in a study done on six patients who were diagnosed with WHS in order to assess the otologic manifestations, some of the patients were having cleft lip and palate, preauricular pits, notched auricles, and branchial clefts sinus tract. Abnormal shape and size of the external ear were identified as well [3].
Management of WHS cases is supportive. However, a multidisciplinary team must be involved in order to coordinate health and care services needed for individuals suffering from this condition [4].
Conclusion
Serious chromosome imbalances can occur as the result of 4p deletion on chromosome 4. For instance, t(4;8) (p16;p23) translocation which characterizes WHS. This syndrome is confirmed by the heterozygous detection of Wolf Hirschhorn critical region (WHCR) [4].
Various facial features are noticed in patients with WHS in addition to the varying intellectual disability from mild to severe depending on how large is the deletion.
Multidisciplinary team is definitely needed to optimize the health care provided to these patients. Furthermore, genetic counselling will perpetuate the patient’s families with information about the genetic disorder, the essence of it, and the proper implications [4].
References
- Battaglia A, Carey J, South S (2015) Wolf-Hirschhorn syndrome: A review and update. American Journal of Medical Genetics Part C: Seminars in Medical Genetics 169(3): 216-223.
- Bulak H, Kopanska D (2017) Wolf–Hirschhorn syndrome – a case report. Pediatria i Medycyna Rodzinna 13(2): 267-269.
- Lesperance M, Grundfast K, Rosenbaum K (1998) Otologic Manifestations of Wolf-Hirschhorn Syndrome. Archives of Otolaryngology–Head & Neck Surgery 124(2): 193-196.
- Rezai S, Wilansky J, Gottimukkala S, Chadee A, Rajegowda BH, et al. (2016) Wolf-Hirschhorn Syndrome (WHS), A Case Report and Review of Literature. Journal of Pediatrics & Neonatal Care 5(1): 5.
- Morgan A, Lenarduzzi S, Spedicati B, Cattaruzzi E, Murru F, et al. (2020) Lights and Shadows in the Genetics of Syndromic and Non-Syndromic Hearing Loss in the Italian Population. Genes 11(11): 1237.
- Paradowska Stolarz A (2014) Wolf-Hirschhorn Syndrome (WHS) – Literature Review on the Features of the Syndrome. Advances in Clinical and Experimental Medicine. 23(3): 485-489.
- Dietze I, Fritz B, Huhle D, Simoens W, Piecha E, et al. (2004) Clinical, Cytogenetic and Molecular Investigation in a Fetus with Wolf-Hirschhorn Syndrome with Paternally Derived 4p Deletion. Fetal Diagnosis and Therapy 19(3): 251-260.
- Aquino S, Machado R, Paranaíba L, Coletta R, Aguiar M, et al. (2015) Uncommon Oral Cleft in Wolf-Hirschhorn Syndrome. Brazilian Dental Journal 26(2): 203-206.






























