5-Fluorouracil (5-FU)-Induced STEMI
Moamen Al Zoubi1*, Divya Korpu2, Tommy Kwak3 and Boddu Prajwal4
1Division of Infectious Diseases and Immunology, University of Massachusetts Memorial Medical Center, USA
2Department of Internal Medicine, Advocate Illinois Masonic Medical Center, USA
3Department of Internal Medicine, Presence Resurrection Medical center, USA
4Department of Leukemia, University of Texas, USA
Submission: August 11, 2017; Published: September 14, 2017
*Corresponding author: Moamen Al Zoubi, Division of Infectious Diseases and Immunology, University of Massachusetts Memorial Medical Center, Worcester MA, USA, Email: Moamen.alzoubi@gmail.com
How to cite this article: Moamen Al Zoubi, Divya Korpu, Tommy Kwak and Boddu Prajwal. 5-Fluorouracil (5-FU)-Induced STEMI. JOJ Case Stud. 2017; 4(2) : 555632. DOI:10.19080/JOJCS.2017.02.555632.
Abstract
Treatment of solid tumor malignancies involves the use of toxic chemotherapeutic agents with potentially life threatening complications and requires vigilance on the part of the physician in promptly recognizing potential adverse effects. We describe a 39 year-old Korean male with history of poorly differentiated gastric adenocarcinoma, who presented with chest pain and shortness of breath 12 hours after completing the first 5-FU cycle. His ECG showed inferior-posterior STEMI. Troponin peaked at 39. He was started on nitroglycerin and heparin drip. His emergent angiography revealed diffusely ectatic coronary arteries. Intracoronary nitroglycerin injection resulted in almost complete resolution of stenosis. He was discharged on aspirin, clopidogrel, atorvastatin, isosorbide mononitrate and amlodipine. It was decided to avoid treatment with 5-FU for his gastric cancer in the future.
Case Presentation
A 39 year-old Korean male with history of poorly differentiated gastric adenocarcinoma, stage IIA (pT2N0M0) status post total gastrectomy and distal esophagectomy who completed the first 5-FU cycle 12 hours prior the presentation to our hospital for chest pain and shortness of breath with one hour duration. He received 5-FU as a continuous IV infusion for 46 hours. Chest pain was substernal, pressure-like, and constant and associated with diaphoresis. ECG showed inferior-posterior STEMI (Figure 1).
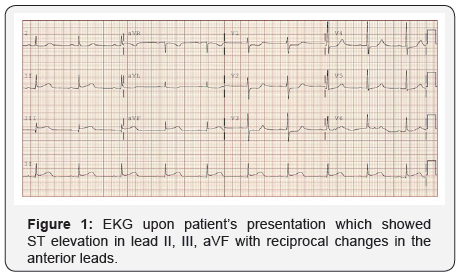
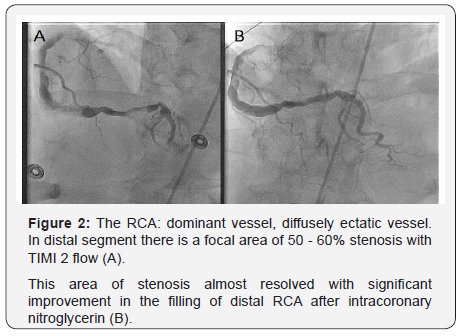
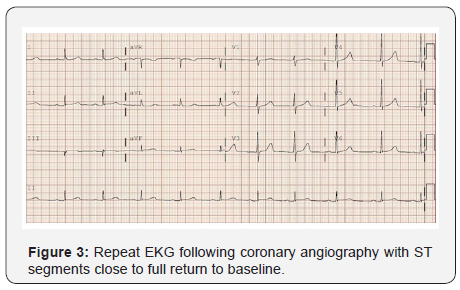
Patient was started on nitroglycerin and heparin drip. A limited echocardiography revealed an ejection fraction of 45-50% with severe hypokinesis of the basal and mid infero-lateral myocardium. He was taken emergently to the catheterization lab for coronary angiography where he was found to have diffusely ectatic coronary arteries (Figure 2). The distal RCA was found to have a focal area with 50-60% stenosis without any evidence of thrombus, which was thought to be the culprit lesion based on the EKG and echocardiographic findings. After intracoronary nitroglycerin injection there was almost complete resolution of stenosis with significant improvement in the filling of the RCA distal segments. Post angiography, patient was continued on nitroglycerin with complete resolution of symptoms and repeated ECG 3 hours later showed almost complete resolution of ST elevation (Figure 3). Troponin peaked at 39. He was also found to have an intermediate lesion in the proximal LAD that was assessed 3 days later with a Lexiscan stress test which was negative for ischemia or infarct areas. Given diffuse coronary ectasia, he was evaluated for collagen vascular disease or Kawasaki disease but workup was negative and he did not meet criteria for complete or incomplete Kawasaki. He was discharged on aspirin 81mg once daily, clopidogrel 75mg once daily, atorvastatin 40mg once daily, isosorbide mononitrate 60mg every morning, and amlodipine 10mg once daily. It was decided to avoid treatment with 5-FU for his gastric cancer in the future.
Discussion
Cardiotoxicities have been reported from the use of 5-FU including acute coronary syndrome, vasospastic angina, coronary thrombosis and dissection, malignant arrhythmias, cardiomyopathy, and sudden cardiac death [1-7]. However, 5-FU has a proven therapeutic benefit and remains an important component of first line adjuvant chemotherapeutic regimens in the management of gastrointestinal malignancies. Hence, careful consideration should be given before discontinuing 5-FU at the cost of decreased therapeutic efficacy of chemotherapy in survival outcomes.
5-FU and its oral prodrug capecitabine are widely used in chemotherapeutic regimens in the treatment of gastrointestinal malignancies. Cardiotoxicities are infrequent but important side effects and may necessitate discontinuation of these medications. Reported incidence of symptomatic cardiotoxicities occur from 1 to 19 percent of patients treated with 5-fluorouracil with severe cardiac events including MI, cardiogenic shock, sudden cardiac death occurring in about 2% of treated patients [8]. Cardiotoxicity was most common during the first cycle of 5-FU chemotherapy [9]. ECG abnormalities during 5-FU administration suggest myocardial ischemia as a plausible mechanism behind most of its cardiotoxic effects [10]. Concept of vasospasm leading to myocardial ischemia has been suggested, since coronary angiography usually failed to show coronary artery stenosis with 5-FU cardiotoxicity [11- 17] although coronary artery vasospasm was observed during coronary angiography in rare cases [18-20].
Several suppositions of 5-FU related coronary vasospasm have been proposed. One theory of 5-FU related coronary vasospasm is higher level of endothelin-1 (ET-1) in 5-FU treated patients. ET-1 is a potent vasoconstrictor produced predominantly by endothelial cells [21,22] But under pathophysiological conditions, production of ET-1 is stimulated in several different cells, including vascular smooth muscle cells, cardiac myocytes, cardiac fibroblasts, macrophages, and leukocytes [21-25]. One study showed higher ET-1 level in patients experiencing 5-FU cardiotoxicity during 5-FU treatment than those without cardiotoxicity [26]. This study also showed higher plasma levels of ET-1 in 5-FU treated patients compared with those receiving non-5-FU chemotherapy [26].
Another theory of 5-FU related coronary vasospasm is protein kinase-C (PK-C) mediated vasoconstriction of vascular smooth muscle. Mosseri et al. [4] used ring of aorta from rabbits exposed to different concentrations of 5-FU and pretreated with PK-C inhibitor and observed for vasoconstriction. The result indicated that 5-FU causes direct, endothelin-independent vasoconstriction of vascular smooth muscle in vitro, this vasomotor response involves activation of PK-C, and this response is independent of vasoactive cell membrane receptors, phosphoinositide turnover, or activation of the cyclooxygenase pathway [4].
Coronary artery ectasia predisposes to complications including coronary artery thrombosis, embolism, and vasospasm [27,28]. Contraintuitive to medical reasoning that ectactic vessels are less capable of spasm due to medial damage, ectatic vessels are actually prone to increased risk for spasm. This was elegantly demonstrated by Bove and Vilestra in a provocation experiment with ergonovine which showed augmented 65 to 93% maximal narrowing of ectactic coronary vessels in the study patients compared to 40% maximal reduction in luminal diameter in normal controls [29]. This narrowing was later shown by computer analysis to occur adjacent to the ectactic portions of the coronary vessel and not in the ectactic portions per se [30]. Sorrell et al. [31] suggested a mechanism linking chronic increased local nitric oxide(NO) production to the development of coronary ectasia, which was later supported by experiments documenting locally increased acetylcholine concentrations from downstream inducible NO synthase (iNOS) pathway stimulation [32]. It may be suggested that the local effects of 5-FU, in our patient, may have provoked the ectactic coronary vessels to go into coronary spasm via increased inhibition of a commonly linked hyperactive iNOS pathway.
Increased vasomotor tone due to inhibition of endothelial NO synthases and endothelium-independent vasoconstriction via protein kinase C pathway potentially explains coronary spasm effects of 5-FU [18]. Traditional cardiac risk factors may increase the risk of cardiac side effects and create diagnostic dilemmas. However, isolated case reports and clinical reviews on patients with 5-FU associated cardiac side effects with no known prior heart disease point towards a true causative cardiotoxic potential of 5-FU [8,10]. Cardiac and renal comorbidities, continuous prolonged duration infusion schedules, concomitant cisplatin treatment are potential risk factors for 5-FU associated cardiotoxicity [1,3,6,8]. There are several case reports which showed vasodilator therapies such as calcium channel blocker and nitrate resulted in resolution of chest pain or normalization of ECG changes [33-37]. Prophylactic calcium channel blockers therapy was successful in one case to prevent recurrence of 5-FU-induced variant angina [36].
Our patient presented within 72 hours of his first cycle administration of 5-FU; upto 70% of the side effects have been noted to occur within the first three days of the first cycle of 5-FU [6]. Diagnosis rests on prompt assessment of chest pain with ECG, cardiac troponins and further angiographic studies if there exists high likelihood for coronary artery thrombosis. Therapy with nitrates and CCBs and discontinuation of 5-FU infusion can result in prompt symptom resolution [38-40]. Currently, there exists insufficient evidence to suggest rechallenges with 5-FU but may be considered if there exist no reasonable alternative therapies in the setting of antivasospastic therapy prophylaxis, close clinical monitoring during infusions and with alternate schedule administrations [3,6,38].
References
- Kosmas C, Kallistratos MS, Kopterides P, Syrios J, Skopelitis H, et al. (2008) Cardiotoxicity of fluoropyrimidines in different schedules of administration: a prospective study. J Cancer Res Clin Oncol 134(1): 75-82.
- Labianca R, Beretta G, Clerici M, Fraschini P, Luporini G (1982) Cardiac toxicity of 5-FU: A study of 1,083 patients. Tumori 68(6): 505-510.
- Jensen SA, Sørensen JB (2006) Risk factors and prevention of cardiotoxicity induced by 5-fluorouracil or capecitabine. Cancer Chemother Pharmacol 58(4): 487-493.
- Mosseri M, Fingert HJ, Varticovski L, Chokshi S, Isner JM (1993) In vitro evidence that myocardial ischemia resulting from 5-fluorouracil chemotherapy is due to protein kinase C-mediated vasoconstriction of vascular smooth muscle. Cancer Res 53(13): 3028-3033.
- Stewart T, Pavlakis N, Ward M (2010) Cardiotoxicity with 5-fluorouracil and capecitabine: more than just vasospastic angina. Intern Med J 40(4): 303-307.
- Saif MW, Shah MM, Shah AR (2009) Fluoropyrimidine-associated cardiotoxicity: revisited. Expert Opin Drug Saf 8(2): 191-202.
- Sorrentino MF, Truesdell AG (2012) 5-Fluorouracil-induced coronary thrombosis: A case report and review of the literature. J Cardiol Cases 6(1): e20-e22.
- Polk A, Vaage-Nilsen M, Vistisen K, Nielsen DL (2013) Cardiotoxicity in cancer patients treated with 5-fluorouracil or capecitabine: a systematic review of incidence, manifestations and predisposing factors. Cancer Treat Rev 39(8): 974-984.
- Robben NC, Pippas AW, Moore JO (1993) The syndrome of 5-fluorouracil cardiotoxicity. An elusive cardiopathy. Cancer 71(2): 493-509.
- Tsibiribi P, Descotes J, Lombard-Bohas C, Barel C, Bui-Xuan B, et al. (2006) Cardiotoxicity of 5-fluorouracil in 1350 patients with no prior history of heart disease. Bull Cancer 93(3): E27-E30.
- Kim SM, Kwak CH, Lee B, Kim SB, Sir JJ, et al. (2012) A case of severe coronary spasm associated with 5-fluorouracil chemotherapy. Korean J Intern Med 27(3): 342-345.
- Tsiamis E, Synetos A, Stefanadis C (2012) Capecitabine may induce coronary artery vasospasm. Hellenic J Cardiol 53(4): 320-323.
- Atar A, Korkmaz ME, Ozin B (2010) Two cases of coronary vasospasm induced by 5-fluorouracil. Anadolu Kardiyol Derg 10(5): 461-462.
- Tajik R, Saadat H, Taherkhani M, Movahed MR (2010) Angina induced by 5-fluorouracil infusion in a patient with normal coronaries. Am Heart Hosp J 8(2): E111–E112.
- Canale ML, Camerini A, Stroppa S, Porta RP, Caravelli P, et al. (2006) A case of acute myocardial infarction during 5-fluorouracil infusion. J Cardiovasc Med (Hagerstown) 7(11): 835-837.
- Mafrici A, Alberti A, Corrada E, Ferrari S, Marenna B (2003) Management of patients with persistent chest pain and ST-segment elevation during 5-fluorouracil treatment: report about two cases. Ital Heart J 4(12): 895-899.
- McGlinchey PG, Webb ST, Campbell NP (2001) 5-fluorouracil-induced cardiotoxicity mimicking myocardial infarction: a case report. BMC Cardiovasc Disord 1: 3.
- Alter P, Herzum M, Soufi M, Schaefer JR, Maisch B (2006) Cardiotoxicity of 5-fluorouracil. Cardiovasc Hematol Agents Med Chem 4(1): 1-5.
- Shoemaker LK, Arora U, Rocha Lima CM (2004) 5-fluorouracil-induced coronary vasospasm. Cancer Control 11(1): 46-49.
- Luwaert RJ, Descamps O, Majois F, Chaudron JM, Beauduin M (1991) Coronary artery spasm induced by 5-fluorouracil. Eur Heart J 12(3): 468-470.
- Levin ER (1995) Endothelins. N Engl J Med 333(6): 356-363.
- Khimji AK, Rockey DC (2010) Endothelin--biology and disease. Cell Signal 22(11): 1615-1625.
- Ito H, Hirata Y, Adachi S, Tanaka M, Tsujino M, et al. (1993) Endothelin-1 is an autocrine/paracrine factor in the mechanism of angiotensin IIinduced hypertrophy in cultured rat cardiomyocytes. J Clin Invest 92(1): 398-403.
- Ehrenreich H, Anderson RW, Fox CH, Rieckmann P, Hoffman GS, et al. (1990) Endothelins, peptides with potent vasoactive properties, are produced by human macrophages. J Exp Med 172(6): 1741-1748.
- Sessa WC, Kaw S, Hecker M, Vane JR (1991) The biosynthesis of endothelin-1 by human polymorphonuclear leukocytes. Biochem Biophys Res Commun 174(2): 613-618.
- Thyss A, Gaspard MH, Marsault R, Milano G, Frelin C, et al. (1992) Very high endothelin plasma levels in patients with 5-FU cardiotoxicity. Ann Oncol 3(1): 88.
- Lange RL, Reid MS, Tresch DD, Keelan MH, Bernhard VM, et al. (1972) Nonatheromatous ischemic heart disease following withdrawal from chronic industrial nitroglycerin exposure. Circulation 46(4): 666-678.
- Carmichael P, Leiben J (1963) Sudden death in explosives workers. Arch Environ Health 7(4): 424-439.
- Bove AA, Vlietstra RE (1985) Spasm in ectatic coronary arteries. Mayo Clin Proc 60(12): 822-826.
- Suzuki H, Takeyama Y, Hamazaki Y, Namiki A, Koba S, et al. (1994) Coronary spasm in patients with coronary ectasia. Cathet Cardiovasc Diagn 32(1): 1-7.
- Sorrell VL, Davis MJ, Bove AA (1996) Origins of coronary artery ectasia. Lancet 347(8995): 136-137.
- Vanhoutte PM (1989) Endothelium and control of vascular function. State of the Art lecture. Hypertension 13(6 Pt 2): 658-667.
- Lestuzzi C, Viel E, Picano E, Meneguzzo N (2001) Coronary vasospasm as a cause of effort-related myocardial ischemia during low-dose chronic continuous infusion of 5-fluorouracil. Am J Med 111(4): 316- 318.
- Gorgulu S, Celik S, Tezel T (2002) A case of coronary spasm induced by 5-fluorouracil. Acta Cardiol 57(5): 381-383.
- Abernethy DR, Schwartz JB (1999) Calcium-antagonist drugs. N Engl J Med 341(19): 1447-1457.
- Kleiman NS, Lehane DE, Geyer CEJ, Pratt CM, Young JB (1987) Prinzmetal’s angina during 5-fluorouracil chemotherapy. Am J Med 82(3): 566-568.
- O’Connell MJ, Martenson JA, Wieand HS, Krook JE, Macdonald JS, et al. (1994) Improving adjuvant therapy for rectal cancer by combining protracted-infusion fluorouracil with radiation therapy after curative surgery. N Engl J Med 331(8): 502-507.
- Lestuzzi C, Crivellari D, Rigo F, Viel E, Meneguzzo N (2010) Capecitabine cardiac toxicity presenting as effort angina: a case report. J Cardiovasc Med (Hagerstown) 11(9): 700-703.
- Farina A, Malafronte C, Valsecchi MA, Achilli F (2009) Capecitabineinduced cardiotoxicity: when to suspect? How to manage? A case report. J Cardiovasc Med (Hagerstown) 10(9): 722-726.
- Spencker S, Schmittel A, Westermann D, Marek A, Schultheiss HP, et al. (2007) [Angina pectoris and ST-elevation after chemotherapy with 5-fluorouracil]. Internist (Berl) 48(1): 69-72.






























