Charcoal and Ozone Treatment Process for the Effluents from Automobile Servicing Stations
Anil K Dwivedi1* and Shashi Dwivedi2
1Department of Botany, Deen Dayal Upadhyay Gorakhpur University, Gorakhpur-273009, India
2Department of Chemistry, St John’s School, Mehrauli, Varanasi-221008, U.P., India
Submission: April 21, 2017; Published: July 11, 2017
*Corresponding author: Anil K Dwivedi, Department of Botany, Deen Dayal Upadhyay Gorakhpur University, Gorakhpur-273009, India, Email: anil.k.dwiwedi@gmail.com
How to cite this article: Anil K D, Shashi D. Charcoal and Ozone Treatment Process for the Effluents from Automobile Servicing Stations. JOJ Case Stud. 2017; 3(4) : 555616. DOI:10.19080/JOJCS.2017.02.555616.
Abstract
Increasing vehicular load has increased the number of automobile servicing stations considerably. The effluent of automobile servicing stations, because of the content of oil and grease is very harmful for the aquatic life. Thus effluents of the automobile servicing stations have been subjected to the charcoal and ozone treatment. Charcoal treatment showed marked reduction in nitrate, phosphate, total hardness etc. in the water samples. But due to highly absorptive and porous nature reduction in DO was also recorded by charcoal treatment. Reduction in DO resulted in increase in BOD and COD. In the case of ozone treatment 100% removal of chromium from polluted water was surprising. Most of the parameters such as acidity, nitrate, phosphate, BOD, COD and total hardness showed reduction by ozone treatment. Ozone treatment also showed increase in the value of DO and total solids. Enormous increase in DO was recorded because ozone gas dissolves in the water and many fold increase in DO was recorded.
Keywords: Ozone treatment; Charcoal treatment; Automobile servicing station; Effluents; Ganga; Varanasi
Introduction
Varanasi is an industrialized city of the country, having more than five thousand large, basic and small scale industries which are situated in and around the city of Varanasi (District Industry Office Records) Manufacturing metal products, chemicals and chemical products, electrical and batter industries, food products, spinning, weaving and finishing of textiles, transport equipments, motor servicing stations, furniture and fixtures, non-metal mineral products, beverages, rubber products including tyre and leather and numerous other products are the prominent. Due to increasing population, coupled by rapid urbanization of the number of automobile servicing stations have increased exponentially in Varanasi. Untreated effluents from these industries containing toxic components, oil and grease, chemicals and other hazardous pollutants are discharged into the nearby water bodies [1], which may be lotic or lentic; ultimately they reach river Ganga directly or indirectly [2].Due to increasing vehicular load the number of automobile servicing station has also increased exponentially, during the last few years. The effluents of automobile servicing station are rich in oil and grease, which affect the surface tension of the water and make a layer over the water surface [3,4]. Thus, the effluent of automobile servicing stations is very much harmful for the flora as well as fauna of the water bodies [5]. The most affected parameter is the oxygen budget, i.e. DO, BOD and COD [6].
Practically feasible and economical treatment procedure for the effluents of automobile servicing station, which may be suitable for Varanasi was needed [7] and this lead to design this research work.
Material and Methods
The study site
Present study was conducted in the cultural capital of India and the holy city Varanasi, which is situated in the eastern part of Uttar Pradesh, a province of India located in core of the Indian subcontinent, lying in the middle of Gangetic plain. On the globe, Varanasi finds its location at 25°18‘ North, 83°1’ East and 76.19 m above the sea level.
The selected research site was near Chaukaghat, which poured the discharge from the Chaukaghat nala. This site was located very close to the old G.T. (Grand Trunk) road. The most common profession in this area was heavy and light vehicles servicing including their washing and painting. Average discharge of Chaukaghat nala was 2.55mld (million liters per day).
Methodology
Potentiometric titration method was used for the determination of acidity of water. Winkler’s modified azide method was used for the estimation of Dissolved oxygen of water (APHA, 1998). Dissolved oxygen of water sample was measured by precipitating as basic manganic oxide, which is dissolved by concentrated sulphuric acid forming manganic sulphate. It immediately reacts with potassium iodide already present, liberating iodine, which is determined by titration with sodium thiosulphate (0.025N).
The basic principle underlying the BOD5 determination is the measurement of the dissolved oxygen content of the sample before and after five days incubation at 20 °C. To measure the dissolved oxygen content of the water samples, Winklers modified azide method was used.
COD is a measure of oxygen equivalent of those constituents in the sample which are susceptible to dichromate oxidation in acidic condition [8]. The known volume of water sample was refluxed with known volume of potassium dichromate and concentrated sulphuric acid for two hours. The remaining amount of potassium dichromate after completing reflux was titrated with ferrous ammonium sulphate using ferroin indicator.
Total hardness of water was estimated by EDTA titrimetric method.
Oil, grease and other extractable matters are dissolved in petroleum ether in the presence of dilute sulphuric acid and separated from the aqueous phase. The solvent layer is then evaporated and the residue is weighed as oil and grease, according to the given equation:
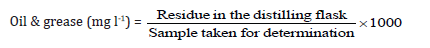
The phenol disulphonic acid method was applied for the analysis for nitrate-nitrogen.
Stannous chloride method was used for the determination of phosphate concentration in water sample.
Total solids of the samples were estimated by evaporating a measured volume of the samples in an oven at 1050C in a dry constant weight crucible. Following formula was used to calculate the total solids.
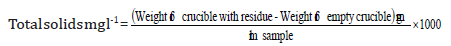
The experiment
50 litre of water sample was maintained in a 100 litre of aquarium. In the setup 10 small packets of tissue paper containing 1 gm activated charcoal each were sinked with the help of weight. Initial readings of all the variables were recorded and the same was analysed 24 hourly for 5 days, as designed by [9,10]. Maximum pollutant removal was recorded on the fourth day. Thus, the value of variables on the 4th day is expressed in the record.
For treatment of the river water two tire bubbling chamber of 12 litre capacity using glass was constructed, whose common surface consisted of profuse perforation, as designed by [11]. The chamber was filled with 10 litre of water sample collected from the polluted study site. Inlet of the lower tire was connected to the modified Siemen-Halske Ozonizer, which consisted of earthed cast iron box and a number of aluminium rods surrounded by glass cylinders cooled by water. The rods are charged to high potential and the air passing through the annular space gets ozonized.
Ozonized gas was passed through the bubbler which comes above, crossing the water in the form of minute bubbles. Change in the parameters was recorded hourly and the maximum pollution removal was found after 6 hours treatment, thus, only this value is expressed in the record.
Result and Discussion
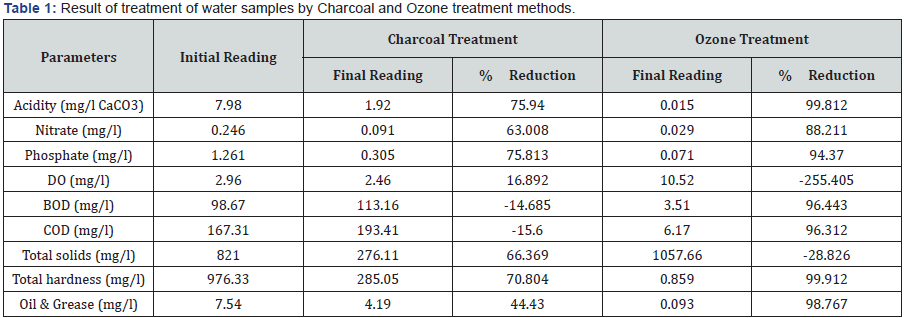
Table 1 Results of the treatments, along with the percentage change in the parameters have been shown in the table 1. Change in all the parameters have been recorded, similar to the findings of [12]. Reduction in total hardness, acidity and nitrate by more than 70% in charcoal treatment is a good response; at the same time the ozone treatment was found to be more affective [13- 16]. Depending on the nature of pollutants, activated charcoal and ozone treatment was supposed to be the most suitable chemical treatment practice. Charcoal treatment showed marked reduction in nitrate, phosphate, total hardness etc. in the water sample, similar to the findings of [17]. But due to highly absorptive and porous nature reduction in DO was also recorded by charcoal treatment, as also reported by [18]. Reduction in DO resulted in increase in BOD and COD, parallel to the findings of [9,19,20] These three are very sensitive parameters for the water quality, thus, this recorded as a prominent drawback of this treatment.
Reduction in acidity, nitrate, phosphate, BOD, COD and total hardness was recorded. Ozone treatment also showed increase in the value of DO and total solids. Enormous increase in DO was recorded because ozone gas dissolves in the water and many fold increase in DO was recorded [21]. The reason for increase in total solids may be due to precipitation of all the pollutants in the form of oxides as a result the amount of total solids by this treatment would have increased. Except only one drawback i.e. increase in the total solids, the ozone treatment was most convincing treatment process [22-24].
Conclusion
Charcoal treatment showed marked reduction in nitrate, phosphate, total hardness etc. in the water sample. But due to highly absorptive and porous nature reduction in DO was also recorded by charcoal treatment. Reduction in DO resulted in increase in BOD and COD. These three are very sensitive parameters for the water quality, thus, this was recorded as a prominent drawback of this treatment.
Reduction in acidity, nitrate, phosphate, BOD, COD and total hardness was recorded. Ozone treatment showed significant increase in the value of DO and total solids.
Acknowledgement
The authors are thankful to Prof. B.D. Tripathi, Coordinator, Environmental Science and Technology, Banaras Hindu University, Varanasi for his valuable suggestions during experimental studies.
References
- Shashi, Dwivedi AK (2009) Ecological Backlashes of Wetland Destruction. Everyman’s Science XLIV 4: 247-249.
- Singh PN, Dwivedi AK (2016) Cultural Importance and Livelihood Contribution of Turanala and Talkandla Wetlands in Gorakhpur, India. Int J Waste Resour 6(3): 245.
- Srivastava G, Kapoor S, Dwivedi AK (2016) Phytoremediation of Water Contaminated by Effluents of Motor Servicing Centre using Hydrilla verticillata and Azolla pinnata. International J of Research in Engineering and Biosciences 3(4): 15-20.
- El-Rehaili AM (1995) Response of BOD, COD and TOC of secondary effluents to chlorination. Water Research 29(6): 1571-1577.
- Udai Bhan P, Dwivedi AK (2016) Free Oxygen Budget of a Polluted Tropical River. Hydrol Current Res 7(2).
- Ice G, Sugden B (2003) Summer Dissolved Oxygen Concentrations in Forested Streams of Northern Louisiana. Southern Journal of Applied Forestry 27(2): 92-99.
- Prajapati UB, Anil K Dwivedi, Singh S (2013) Sustainable Utilisation of Aquatic Macrophyte for Waste Water Treatment. Advances in Plant Sciences 26(2): 443-446.
- Gajghate DG, Reddy PJ (1989) COD-BOD relationships for Industrial Wastes. Indian Journal of Environment Protection 9(11): 805-807.
- Singh JS, Dwivedi AK (2010) Numerical analysis of DO, BOD and COD in a polluted tropical river. Columban Journal of Life Sciences 11(1&2): 19-24.
- Bachmann V, Ussieglio PP (1999) Contribution of the macrobenthic compartment to the oxygen budget of a large regulated river:the Mosel. Hydrobiology 410: 39-46.
- Shashi, Dwivedi AK (2010) River Pollution and Solution. New Central Book Agency (P) Ltd., Calcutta, India.
- Rani PS, Reddy PM (2004) Comparative study of physico-chemical and phycological properties of Hussain-Sagar Lake. Journal of Industrial Pollution Control 20(2): 193-198.
- Rao CS (2006) Environmental Pollution Control Engineering. New Age International (P) Limited Publishers New Delhi, India, pp. 295-297.
- Singh KP, Malik A, Mohan D, Sinha S (2004) Multivariate statistical techniques for the evaluation of spatial and temporal variations in water quality of Gomti River (India)--a case study. Water Res 38(18): 3980-3992.
- Singh KP, Malik A, Mohan D, Sinha S (2005) Water quality assessment and apportionment of pollution sources of Gomti river (India) using multivariate statistical techniques-a case study. Analytica Chimica Acta 538 (1-2): 355-374.
- Singh KP, Malik A, Singh VK, Basant N, Sinha S (2006) Multi-way modeling of hydro-chemical data of an alluvial river system--A case study. Anal Chim Acta 571(2): 248-259.
- Shashi, Singh J, Dwivedi AK (2009) Numerical interdependence of pH, acidity and alkalinity of a polluted river water. J Environ Biol 30(5): 773-775.
- Chen GH, Leong TM, Liu J, Huang JC (1999) Study of oxygen uptake by tidal river sediment. Water Research 33(13): 2905-2912.
- Jana BB, Sengupta S (1989) Responses of dissolved oxygen, biochemical and chemical oxygen demands of water to artificial aeration. International Journal of Environmental Studies 33(4): 307-315.
- Boyd CE, Romaire RP, Johnston E (1978) Predicting Early Morning Dissolved Oxygen Concentrations in Channel Catfish Ponds. Transactions of the American Fisheries Society 107(3): 484-492.
- Tiwari TN, Das SC, Bose RK (1986) A relation between COD and BOD for the Ganga at Kanpur. Indian Journal of Environment Protection 6(3): 183-184.
- (1998) Standard Methods for Examination of Water and Wastewater. In:American Public Health Association, American Water Works Association, and Water Pollution Control Federation. (20th edn), Washington, USA.
- Dwivedi AK, Shashi, Singh J (2006) Water pollution and groundwater recharging. Current Science 91(4): 407-408.
- Dwivedi AK, Prajapati UB, Shashi (2007) Waste Water and its Management. Indian Science Cruiser 21(4): 36-40.






























