An Unusual Case of Tachy-Brady Syndrome Caused by Cholecystitis
Paige Bell1*, Ari Shaeffer2and Gerald OóMalley3
1Medical Student, Grand Strand Medical Center, USA
2Emergency Medicine Resident, Grand Strand Medical Center, USA
3Director of Toxicology, Grand Strand Medical Center, USA
Submission: March 07, 2018; Published: May 08, 2018
*Corresponding author: Paige Bell, OMS IV Medical Student, Emergency Medicine Residency Program, Grand Strand Medical Center, 809 82nd Pkwy, Myrtle Beach, SC 29572, USA, Tel: 561-306-5304; Email: pbell@vcom.edu
How to cite this article: Paige B, Ari S, Gerald O. An Unusual Case of Tachy-Brady Syndrome Caused by Cholecystitis. J Cardiol & Cardiovasc Ther.2018;10(3): 555788. DOI: 10.19080/JOCCT.2018.10.555788
Abstract
Purpose: To describe an unusual case of wide complex tachycardia associated with vagus nerve dysfunction caused by cholecystitis.
Case report:71-year-old female presented to the emergency department with three days of atypical chest pain associated with right sided abdominal pain. Electrocardiogram demonstrated wide complex tachycardia resistant to multiple interventions. Ultrasound revealed cholecystitis. Subsequent to percutaneous cholecystostomy the cardiac rhythm devolved from recalcitrant tachycardia to tachy-brady syndrome. A dual-chamber pacemaker was installed followed by elective cholecystectomy. The patient was discharged home in stable condition.
Conclusion:This case illustrates an unusual cause of cardiac dysfunction via vagus nerve mediated gastrointestinal inflammatory process.
Keywords: Tachy-brady syndrome; Atrial fibrillation/flutter; Sick sinus syndrome; Cholecystitis
Introduction
Tachy-brady syndrome is a form of sick sinus syndrome most often caused by inability of the sinoatrial (SA) node to generate an appropriate rate. The pacemaking ability of the SA node is not completely understood, but it is known that both the parasympathetic and sympathetic nervous systems innervate the SA node. Parasympathetic innervation of the SA node is mediated by the vagus nerve and typically decreases the SA node's pacemaking potential and is more prominent at rest. Vagus nerve dysfunction is an unusual and rarely reported cause of sick sinus syndrome. Vagal contribution to the innervation of the gastrointestinal and cardiovascular systems has been described as linked, but the specific mechanism in which they are associated is hypothetical and not well understood at this time. Typical treatment for tachy-brady syndrome is implantation of a cardiac pacemaker for rate control.
We present a case of sick sinus syndrome, wide-complex recalcitrant atrial tachycardia and tachy-brady syndrome that we believe to be caused by acute cholecystitis.
Case Report
A 71-year-old female with past medical history of hypothyroidism and arthritis presented to the emergency department with three days of upper chest and throat tightness that began suddenly while walking. The chest pain was associated with progressive fatigue and shortness of breath. She also reported diaphoresis and abdominal pain with decreased appetite when her chest discomfort was at its worst. On arrival to the emergency department, the patient was experiencing dizziness, nausea, and epigastric pain that radiated to the left abdomen. The patient denied fevers, chills, vomiting, and dysuria on presentation.
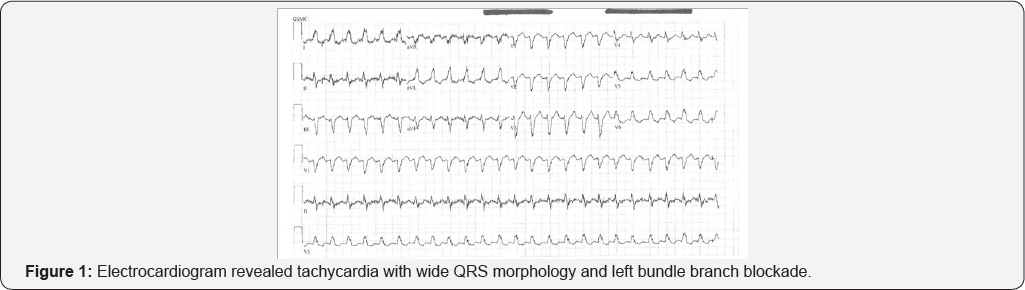
In the emergency department, the patient's vital signs included a temperature of 98.7 degrees Fahrenheit (oral), pulse of 149 beats per minute, blood pressure of 151/98mmHg, respiratory rate of 24 breaths per minute, and a pulse oximetry reading of 96% on room air. Physical examination revealed an ill-appearing Caucasian female with tachycardia without unusual heart sounds and slight epigastric tenderness without guarding or rebound tenderness. Electrocardiogram (Figure 1) revealed tachycardia with wide QRS morphology and left bundle branch blockade. Pertinent laboratory results included white blood cell count of 13.5K/mm3 and initial troponin of .08ng/mL.
Aspirin, oxygen, nitroglycerin and an amiodarone bolus of 150mg were administered. The patient's heart rate remained around 140 beats per minute but blood pressure dropped to 80/50mmHg. At this point, diltiazem 25mg, was administered in 5mg boluses at 10 minute intervals, interspersed with intravenous saline in attempts to slow her heart rate and allow time for her blood pressure to normalize. Eventually the patient's heart rate slowed to approximately 60-90 beats per minute, and her blood pressure increased to 100/80mmHg. The patient remained awake and alert and was admitted to the intensive care unit with cardiology consultation.
Once stabilized in the intensive care unit, aggressive cardiac rate control continued with amiodarone, diltiazem and metoprolol infusion and digoxin bolus therapy, however the patient s heart rate remained greater than 140 beats per minute. At one point, the patient's wide complex tachydysrhythmia converted to atrial flutter and a heparin infusion was begun due to concern for cardiac ischemia. Echocardiogram revealed no serious wall motion abnormalities and an ejection fraction of 55-60% at this time.
Throughout her stay in the hospital the patient continued to complain of nausea and anorexia and her abdominal pain localized to the right side. An ultrasound revealed an abnormal gallbladder with significant wall thickening, edema, and pericholecystic fluid with stones concerning for acute cholecystitis. Percutaneous gallbladder drainage was performed and while in the recovery room, the patient's heart rate changed from tachycardia to sinus bradycardia at 40 beats per minute. All parenteral medications were immediately withdrawn as to not further decrease her heart rate. Several hours later, the patient again developed atrial fibrillation with ventricular rate of 150 beats per minute. A dualchamber pacemaker and electric external cardioversion device was placed and the following day, the patient converted back to normal sinus rhythm.
Following successful cholecystectomy on hospital day eight, the patient was discharged home with metoprolol, amiodarone, diltiazem, aspirin and rivaroxaban in stable condition.
Discussion
Gastrointestinal pathology causing cardiac dysrhythmias has been described, though not to the same extent as the converse.
Anatomically the esophagus and cardiac atria are adjacent and have similar vagal nerve innervation. The association between atrial fibrillation and gastric reflux is well described, but the exact relationship is hypothetical and thought to involve vagus nerve parasympathetic overstimulation of the gastrointestinal and cardiac muscles with resultant dysfunction of both [1].
The Vagus nerve is one of the most widely distributed cranial nerves, carrying sensory information from and parasympathetic fibers to the heart, respiratory, and gastrointestinal tract. The vagus nerve originates in the medulla, then exits the cranium through the jugular foramen between the glossopharyngeal nerve and the internal jugular vein. The nerve then continues down the neck within the carotid sheath giving off many branches as it travels throughout the body. Along the nerve, efferent, afferent, and parasympathetic fibers send information to and from the brain. Vagus innervation beyond the gastrointestinal tract is very diffuse, innervating organs such as the stomach, superior duodenum, liver, gallbladder, pancreas, inferior duodenum, and the first two-thirds of the transverse colon. Although efferent fibers from the gut are essential for gastric motility and secretion, it is estimated that 90% of vagal fibers from the gut are afferent [2].
Atrial ablation frequently results in functional impairment of the upper gastrointestinal system [3]. The impaired peristalsis is transient and most likely due to injury or interruption of vagal nerve conduction. Whether interruption or disruption of gastrointestinal vagal stimulation causes cardiac dysrhythmia or dysfunction is unclear [3]. The relationship between gastric and cardiac vagal innervation is currently not well defined [2,3]. Cardiac complications, atrial fibrillation in particular, is a well- described postoperative risk following esophageal surgery [4].
Gallbladder innervation includes sympathetic stimulation via the celiac plexus, parasympathetic innervation supplied by the vagus nerve and unmyelinated segmental afferent fibers conveying sensory information. Cholecystitis is inflammation of the gallbladder usually due to an obstructing stone. Increased intraluminal pressure and distention of the gallbladder causes stretching of unmyelinated sensory fibers resulting in pain, nausea and vomiting. Stretch or distention of the gallbladder is the principal stimulus involved in visceral pain.
Visceral afferent nerves follow segmental distribution, localized by the sensory cortex to a specific spinal segmental level. Most visceral pain stemming from organs that are innervated bilaterally presents with diffuse poorly localized pain. Although the gallbladder is bilaterally innervated through nociceptive inputs from the spinal segments of T5-T10 it is an exception to this generalization; the pain associated with gallbladder dysfunction and inflammation is more localized to the right, due to the fact that it is predominantly innervated on its ipsilateral side [5].
A recent Japanese report describes the presentation of a series of patients with cholecystitis that were initially misdiagnosed as having primary cardiovascular disease [6]. It is reasonable to surmise that gallbladder inflammation could transmit pain via segmental nociceptive afferent fibers, just as it could communicate via its vagal afferent fibers, affecting the vagal pathway and inducing cardiac dysrhythmia as was seen with our patient.
References
- Floria M, Barboi O, Rezus C, Ambarus V, Cijevschi-Prelipcean C, et al. (2015) Atrial fibrillation and gastro-oesophageal reflux disease -controversies and challenges. Curr Pharm Des 21(26): 3829-3834.
- Camara R, Griessenauer C (2015) Anatomy of the vagus nerve. In Nerves and Nerve Injuries (1st edn), MA: Academic Press, Cambridge, USA 1: 385-397.
- Lakkireddy D, Reddy YM, Atkins D, Rajasingh J, Kanmanthareddy A, et al. (2015) Effect of atrial fibrillation ablation on gastric motility: the atrial fibrillation gut study. Circ Arrhythm Electrophysiol 8(3): 531536.
- Chebbout R, Heywood EG, Drake TM, Wild JRL, Lee J, et al. (2017) A systematic review of the incidence of and risk factors for postoperative atrial fibrillation following general surgery. Anesthesia 73(4): 490-498.
- Tintinalli J, Stapczynski J, Ma O, Yealy D, Meckler G, et al. (2015) Tintinalli's emergency medicine: a comprehensive study guide (8th edn), NY: McGraw-Hill Education, New York, USA.
- Ozeki M, Takeda Y, Morita H, Miyamura M, Sohmiya K, et al. (2015) Acute cholecystitis mimicking or accompanying cardiovascular disease among Japanese patients hospitalized in a Cardiology Department. BMC Res Notes 8: 805.






























