Successful Treatment of Cardiomyopathy Induced by Premature Ventricular Complexes
Nandakumar Ramakrishna*, Koh Kok Wei, Alan Koay Choon Chern, Yap Lok Bin, Razali Omar and Zulkeflee Muhammad
Department of Cardiology (Electrophysiology Unit), National Heart Institute, Malaysia
Submission: December 18, 2017; Published: January 19, 2018
*Corresponding author: Nandakumar Ramakrishnan, Department of Cardiology, National Heart Institute (IJN), Malaysia, Email: sainte_13@hotmail.com
How to cite this article: Nandakumar R, Koh K W, Alan K C C, Yap L B, Razali O, Zulkeflee M. Successful Treatment of Cardiomyopathy Induced by Premature Ventricular Complexes. J Cardiol & Cardiovasc Ther 2018; 9(1): 555755. DOI: 10.19080/JOCCT.2018.09.555755
Abstract
Background: Idiopathic premature ventricular complexes (PVCs) are considered a benign form of cardiac arrhythmia. However, several reports demonstrated that frequent PVCs were associated with left ventricular (LV) dysfunction, increased LV dimensions and cardiomyopathy.
Case Report: A 52-year-old lady with underlying diabetes mellitus, hypertension and dyslipidemia presented with dyspnea and palpitation. Echocardiography showed ejection fraction (EF) of 25% with LV systolic diameter 5.2cm and mild functional mitral regurgitation (MR). Holter revealed 13657 isolated and couplet PVCs which represented 11.2% of total QRS count in 24 hours. Cardiac MRI showed no inducible ischemia, fibrosis, infarction or inflammation. Her baseline 12-lead ECG showed inferior axis PVCs with left bundle branch block morphology. Electrophysiology study using the Carto® 3 System (Biosense Webster, Irvine, CA) showed PVCs arising from anteroseptal right ventricular outflow tract. Based on earliest activation and QS signal on unipolar channel together with pace-mapping, PVCs were successfully ablated. Since then she was symptom-free and a repeat Holter after 3 months showed reduced PVC burden (0.3% over 24hours). Echocardiogram showed an improved EF of 51% with LV systolic diameter 3.8cm and disappearance of MR.
Conclusion: Frequent idiopathic PVCs can lead to cardiomyopathy, which can be potentially reversed with catheter ablation
Keywords: Premature ventricular complexes; Cardiomyopathy; Ablation
Introduction
It was previously thought that frequent ventricular complexes in patients without structural heart disease was considered to be a benign condition without prognostic significance [1,2]. However, Duffee et al. [3] in 1998 had shown that premature ventricular complexes (PVCs) was associated with cardiomyopathy and pharmacological suppression of PVCs in patients with presumed idiopathic dilated cardiomyopathy subsequently improved left ventricular (LV) systolic dysfunction.
Several other case reports suggest that frequent PVCs might cause a cardiomyopathy that is reversible by elimination of the PVCs [4-6]. Furthermore, several studies have shown that frequent PVCs can result in a reversible form of cardiomyopathy [7-10]. This case report illustrates and substantiates that the elimination of PVCs with radiofrequency ablation leads to reversal of dilated cardiomyopathy.
Case Report
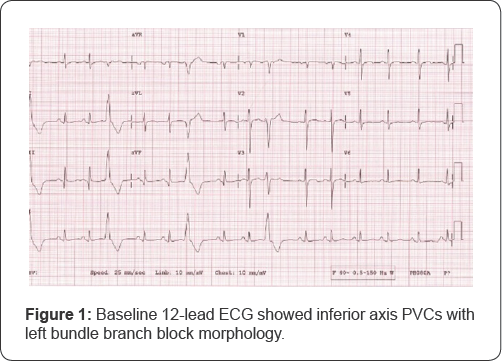
A 52-year-old lady with underlying diabetes mellitus, hypertension and dyslipidemia presented with dyspnea and palpitation for one year duration. She was diagnosed with dilated cardiomyopathy based on echocardiography that showed a reduced left ventricular ejection fraction (LVEF) of 25% and LV systolic diameter 5.2cm with mild functional mitral regurgitation (MR). Her baseline 12-lead ECG showed inferior axis PVCs with left bundle branch block morphology (Figure 1). Holter recording showed that 13,657 isolated PVCs occurred in a 24-hour period with a uniform, inferior-axis morphology representing 11.2% of the total QRS count. Cardiac magnetic resonance imaging did not show inducible ischemia, fibrosis, or inflammation. She was started on anti-heart failure medications and despite optimization of medical therapy, she still remained symptomatic.
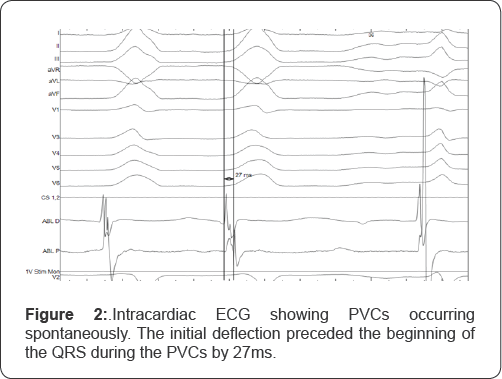
Electrophysiology study was conducted using Carto® 3 System (Biosense Webster, Irvine, CA). A 10-pole catheter (6 French, Auto ID, Biosense Webster) was introduced into the coronary sinus as a reference point via the right femoral vein. Frequent PVCs were induced with a bolus of isoprenaline. Activation mapping with a contact-force ablation catheter (7.5 French, ThermoCool® SmartTouch® Catheter, Biosense-Webster) was performed and the earliest activation was detected from the anteroseptal right ventricular outflow tract (Figure 2).
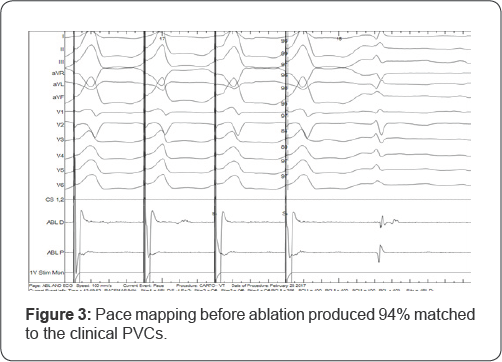
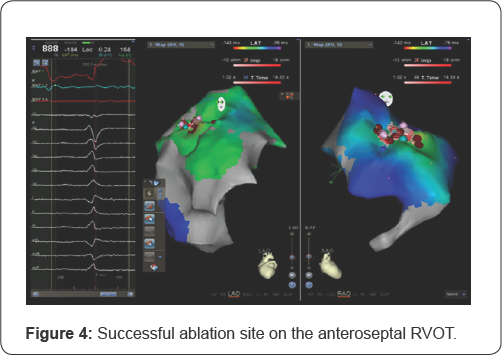
Pace mapping at this location reproduced the morphology of PVC in 11 of the 12 ECG leads (Figure 3) and QS signal in unipolar (Figure 4). Radiofrequency ablation (RFA) was applied to this site with energy up to 35W at 43 °C for 60 seconds and the PVCs disappeared. At the end of the procedure, no PVCs were elicited even with the infusion of isoprenaline. The patient was discharged well and reviewed after 3 months with echocardiography and Holter. Her symptoms resolved and she was now in NYHA functional class 1. The echocardiography showed an improved EF of 51%, with LV systolic diameter 3.8cm and disappearance of MR.
Discussion
This case report is another example of a patient who was diagnosed with dilated cardiomyopathy without structural abnormality but had frequent PVCs. She had successfully undergone PVC catheter ablation, which resulted in resolution of symptoms and complete recovery of EF, LV dimension and MR.
An association between frequent ventricular arrhythmias and LV dysfunction has been documented [11,12]. However only recently Duffee et al. [3] showed a causal relationship between frequent ventricular ectopy and a reduced EF; and demonstrated an improvement in LV function with suppression of ventricular ectopy by anti-arrhythmic drug therapy. Chugh et al. [4] published the first case report of a cardiomyopathy that was reversed by focal ablation of the source of isolated PVCs. Furthermore, two recent studies demonstrated that enlarged LV dimensions decreased after successful ablation of frequent predominantly right ventricular ectopy [7,8]. Yarlagadda et al. [9] showed that frequent ectopy originating from the right ventricular outflow tract could cause a reversible form of cardiomyopathy. The mechanism by which frequent PVCs result in LV dysfunction is unclear but may involve ventricular dyssynchrony or an increase of oxygen consumption, as demonstrated by other investigators [13,14].
Since the first report of improvement of cardiomyopathy following successful ablation of PVCs by Chugh et al. [4], radiofrequency catheter ablation has increasingly been used in preference to medical therapy for the treatment of PVCs. Actual PVC burden with regards to quantity and duration leading to cardiomyopathy is still unclear. Further research is required to identify the optimal timing for the initiation of therapy. More studies to determine the involvement of structural or genetic predisposition in the development of PVC-induced cardiomyopathy are warranted. Until more randomized trials are carried out, treatment modality of PVCs should be based on the clinician's discretion.
Conclusion
Frequent idiopathic PVCs can lead to cardiomyopathy, which can potentially be reversed with catheter ablation.
References
- Gaita F, Giustetto C, Di Donna P, Richiardi E, Libero L, et al. (2001) Longterm follow-up of right ventricular monomorphic extrasystoles. J Am Coll Cardiol 38(2): 364-370.
- Conti C (2005) Ventricular arrhythmias: a general cardiologist's as-sessment of therapies in 2005. Clin Cardiol 28(7): 314-316.
- Duffee DF, Shen WK, Smith HC (1998) Suppression of frequent pre-mature ventricular contractions and improvement of left ventricular function in patients with pre-sumed idiopathic dilated cardiomyopa-thy. Mayo Clin Proc 73(5): 430-433
- Chugh SS, Shen WK, Luria DM, Smith HC (2000) First evidence of premature ventricular complex-induced cardiomyopathy: a potentially reversible cause of heart failure. J Cardiovasc Electrophysiol 11(3): 328-329.
- Shiraishi H, Ishibashi K, Urao N, Tsukamoto M, Hyogo M, et al. (2002) A case of cardiomyopathy induced by premature ventricular complexes. Circ J 66: 1065-1067.
- Grimm W, Menz V, Hoffmann J, Maisch B (2001) Reversal of tachycardia induced cardiomyopathy following ablation of repetitive monomor- phic right ventricular outflow tract tachycardia. Pacing Clin Electrophysiol 24(2): 166-171.
- Takemoto M, Yoshimura H, Ohba Y, Matsumoto Y, Yamamoto U, et al. (2005) Radiofrequency catheter ablation of premature ventricular complexes from right ventricular outflow tract improves left ventricular dilation and clinical status in patients without structural heart disease. J Am Coll Cardiol 45(8): 1259-1265.
- Sekiguchi Y, Aonuma K, Yamauchi Y, Obayashi T, Niwa A, et al. (2005) Chronic hemodynamic effects after radiofrequency catheter ablation of frequent monomorphic ventricular premature beats. J Cardiovasc Electrophysiol 16(10): 1057-1063.
- Yarlagadda RK, Iwai S, Stein KM, Markowitz SM, Shah BK, et al. (2005) Reversal of cardiomyopathy in patients with repetitive monomorphic ventricular ectopy originating from the right ventricular outflow tract. Circulation 112: 1092-1097.
- Bogun F, Crawford T, Reich S, Koelling TM, Armstrong W, et al. (2007) Radiofrequency ablation of frequent, idiopathic premature ventricular complexes: comparison with control group without intervention. Heart Rhythm 4(7): 863-867.
- Kennedy HL, Pescarmona JE, Bouchard RJ, Goldberg RJ, Caralis DG (1982) Objective evidence of occult myocardial dysfunction in patients with frequent ventricular ectopy without clinically apparent heart dis-ease. Am Heart J 104(1): 57-65.
- Lemery R, Brugada P, Bella PD, Dugernier T, van den Dool A, et al. (1989) Non-ischemic ventricular tachycardia. Clinical course and long-term follow-up in patients without clinically overt heart disease. Circulation 79: 990-999.
- Chardack WM, Gage AA, Dean DC (1965) Paired and coupled electrical stimulation of the heart. Bull NY Acad Med 41: 462- 480.
- Hoffman BF, Bartelstone HJ, Scherlag BJ, Cranefield PF (1965) Effects of postextra-systolic potentiation on normal and failing hearts. Bull N Y Acad Med 41: 498-534.






























