Abstract
Background: The COVID-19 pandemic prompted a lockdown in early 2020, disrupting cancer services worldwide. We evaluated the impact on diagnosis and management of patients with locally advanced cervical cancer (LACC) referred to a regional center in the West of Scotland.
Methods: The radiotherapy prescribing system identified those commencing primary chemoradiotherapy (CCRT), 1st April 2020 to 31st March 2021. Stage at presentation, treatment pathway components, and relapse/survival outcomes were documented and compared with a previous year.
Results: Fewer patients were referred for CCRT in the post-COVID period (n=75 versus n=82). Based on International Federation of Gynecology & Obstetrics (FIGO) 2018, stage distribution was I (13% versus 4%), II (54% versus 27%), III (25% versus 49%), IV (9% versus 20%), pre-COVID and post-COVID, respectively (p<0.001). Virtually all patients received 45Gy/25#, delivered by Volumetric Modulated Arc Therapy (VMAT), with daily adaptive plan selection. Brachytherapy (or alternative) boost was completed in 96% pre-COVID and 90% post-COVID. Concurrent cisplatin administration rate was 90% pre-COVID and 85% post-COVID. No deviations in CCRT pathway or gaps occurred due to COVID-19 infection. Relapse frequency at one and three years was 8% & 23% and 15% & 29% pre-COVID and post-COVID, respectively. Pelvic and systemic control and the proportion of patients alive and disease-free were all marginally higher in the pre-COVID cohort.
Conclusion: Clinical management of LACC did not alter markedly in the first year of the COVID-19 pandemic. However, we observed a statistically significant pattern of stage migration and outcomes at three years post diagnosis were slightly less favorable.
Keywords: Cervical cancer stage; chemo-radiotherapy; brachytherapy; relapse; survival; COVID-19.
Introduction
The advent of Coronavirus disease (COVID-19) prompted a UK-wide lockdown in March 2020, following the World Health Organization announcement of a global pandemic. Remobilization of health services to facilitate emergency care of unwell patients infected with a novel virus impacted on patient care for non-communicable diseases. Cancer services were disrupted as a result, and outcomes have emerged suggesting reduced survival for various tumor types, such as lung cancer [1]. This is likely multifactorial, reflecting compromised access to diagnostic investigations, delays to definitive management, and/or adaptations to standard treatment (often de-intensified). A systematic rapid review suggested that gynecological cancer practice altered considerably in 2020 [2], but most of the literature is focused on surgery. For example, one in five patients undergoing gynecological cancer surgery experienced treatment modifications during COVID-19 [3]. Significant adverse outcomes were observed in those with delayed or cancelled operations, largely pertaining to endometrial or ovarian cancer. The data are sparse on disruption to cervical cancer management. Yet, as a Category 1 malignancy, cervical cancer remained a key treatment priority. Concurrent chemo-radiotherapy (CCRT) is the internationally accepted gold standard management for locally advanced disease [4], but the safety of this approach at the beginning of the pandemic was unclear. In early 2020, patients with cancer were thought to be particularly vulnerable to COVID-19, most notably if they were receiving cytotoxic chemotherapy [5]. Additionally, there were concerns over safe delivery of protracted RT courses, especially the administration of brachytherapy as this is often facilitated by general anesthesia, and endotracheal intubation was highlighted as a hazardous aerosol generating procedure. In order to direct management, the UK Royal College of Radiologists (RCR) produced a document on gynecological cancer in May 2020 suggesting potential adaptations to best practice that may be considered if resources were to become limited due to COVID-19 [6]. Various international guidelines were also rapidly progressed to aid clinicians making complex treatment decisions under adverse circumstances [7].
Over 250 cases of cervical cancer are diagnosed annually in Scotland [8]. The West of Scotland Cancer Network (WOSCAN) serves just over half of the Scottish population, a significant proportion of whom are socioeconomically deprived, often with associated comorbidities, including obesity, which was recognized to be a risk factor in contracting severe COVID-19 [9]. Up to 100 patients undergo radical CCRT (or RT) for locally advanced cervical cancer in WOSCAN every year. All treatment is delivered at a single institution tertiary referral center with no acute general medical receiving on-site. In order to evaluate the potential impact of the pandemic on diagnosis and the subsequent delivery of CCRT for locally advanced cervical cancer in WOSCAN, we evaluated tumor size and stage for a 12-month period commencing 1st April 2020 and compared with data from a previous year. Also, we describe the frequency and extent of deviations in standard practice at our institution based on resource limitation (and/or concurrent positive COVID-19 Polymerase Chain Reaction (PCR) test). Finally, we present disease control and survival outcomes at three years post diagnosis.
Materials and Methods
Patient group
Patients who commenced radical RT/CCRT in WOSCAN between 1st April 2020 and 31st March 2021 were identified from the central radiotherapy prescribing system (ARIA®). For the purposes of a historical control, 1st April 2018 to 31st March 2019 served as a comparison. Clinical and pathological data were retrieved from corresponding medical records, including information on histological subtype, imaging modality at diagnosis, tumor size (maximum diameter recorded on MRI, or clinical examination if MRI not performed), stage (according to International Federation of Gynecology & Obstetrics (FIGO) versions 2009 and 2018) and treatment details pertaining to external beam radiotherapy (EBRT), image guided brachytherapy (IGBT), and administration of chemotherapy. Statistical analysis was performed using R version 4.4.1 and RStudio 2024.04.2. Wilcoxon rank sum test was used for continuous and discrete variables. Fisher exact test was used for categorical variables.
CCRT regimen
The standard of care at WOSCAN in 2020 consisted of volumetric modulated radiotherapy (VMAT), 45Gy/25#, with weekly concurrent cisplatin 40mg/m2, and IGBT boost, 24Gy/4#. Daily cone beam (CBCT) soft tissue matching is performed to select the most appropriate “plan of the day” from a series of adaptive plans based on predicted organ motion. Simultaneous integrated boost (SIB) is considered for selected patients with involved nodes (up to 55 Gy), and 50 Gy is delivered to central pelvis if primary cervical tumor >5cm. Two brachytherapy insertions are performed (in weeks 5 and 6) with the applicator remaining in situ overnight, permitting two fractions to be delivered at each insertion with a gap of approximately 20 hours. CT scan is used to delineate the organs at risk, and dose is prescribed to Point A as per the Manchester system. In addition, neoadjuvant chemotherapy, consisting of 3-weekly Carboplatin AUC5 / Paclitaxel 175mg/ m2, is often utilized in those with “high-risk” features (primary tumor >5cm, multiple nodes, any node ≥15mm, presence of paraaortic node(s)). Follow up consists of post-treatment MRI at three months followed by clinical examination every three to six months for a minimum of three years (data lock 30th June, 2024). COVID-19 PCR testing was performed 48 hours prior to admission for IGBT.
Results
Patient Demographics
Slightly fewer patients were referred for CCRT in the post- COVID period (n=75) compared with pre-COVID (n=82). Patient demographics are illustrated in (Table 1). Median age was similar across the two groups but body mass index (BMI) was higher (p=0.026) and there was a greater preponderance of non-squamous histology in the post-COVID cohort (p=0.026). The median tumor size was larger post-COVID at 49mm compared with 45mm pre- COVID, but this did not reach statistical significance (p = 0.3). There were more tumors measuring ≥50mm post-COVID and the largest maximum tumor diameter of 130mm was also recorded in this time period. Rates of diagnostic imaging were analogous, indicating that access to accurate staging was not compromised, but median time to start treatment was significantly higher post- COVID (p<0.001).
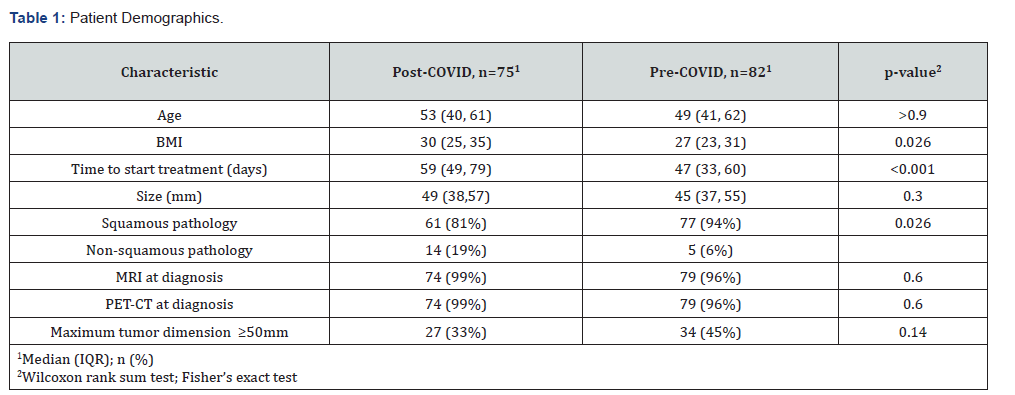
Abbreviations: BMI: body mass index; IQR: interquartile range; MRI: magnetic resonance imaging; PET-CT: positron emission tomographycomputed tomography
Stage at diagnosis
Based on FIGO 2009, more patients presented with earlier stage (I/II) cervical cancer pre-COVID as opposed to post- COVID (87% and 67%, respectively), as shown in (Table 2). After applying the FIGO 2018 system, stage I/II distribution at diagnosis dropped to 67% and 31%, respectively. The presence of nodal involvement, as depicted by the FIGO 2019 IIIC category, was doubled in the post-COVID cohort (42%) compared with the pre-COVID cohort (21%). In addition, the proportion of patients with stage IVA disease was almost three times as high post-COVID (17% versus 6%). A small number of patients with IVB cervical cancer were included as they had small volume bony metastases or peritoneal involvement limited to the pelvis. Overall, there was a statistically significant pattern of stage migration post-COVID (see Supplementary Table 1).
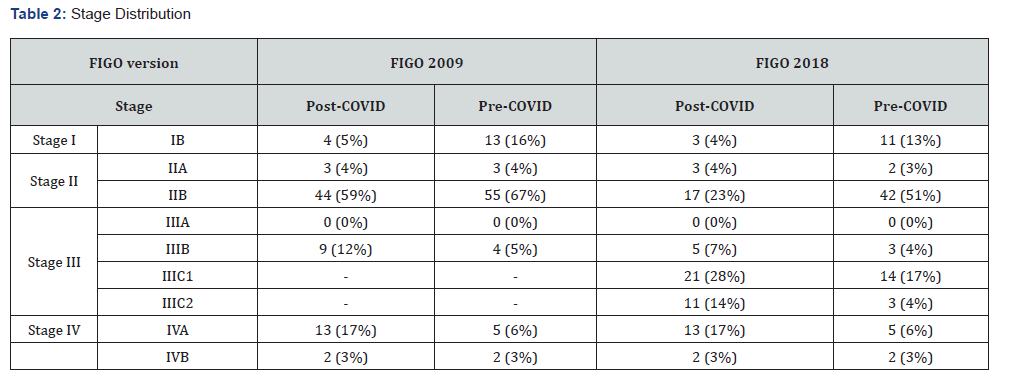
Abbreviations: FIGO – International Federation of Obstetrics & Gynecology
CCRT pathway
Chemotherapy
The rate of concurrent cisplatin administration was 5% lower post-COVID; the primary reason was renal function / comorbidities, and not COVID-19. However, neoadjuvant chemotherapy was delivered to a higher proportion of patients post-COVID (31% versus 21%, respectively). Median number of cycles was 3 (range 1-6) in both cohorts. (Table 3) illustrates each component of the CCRT pathway.
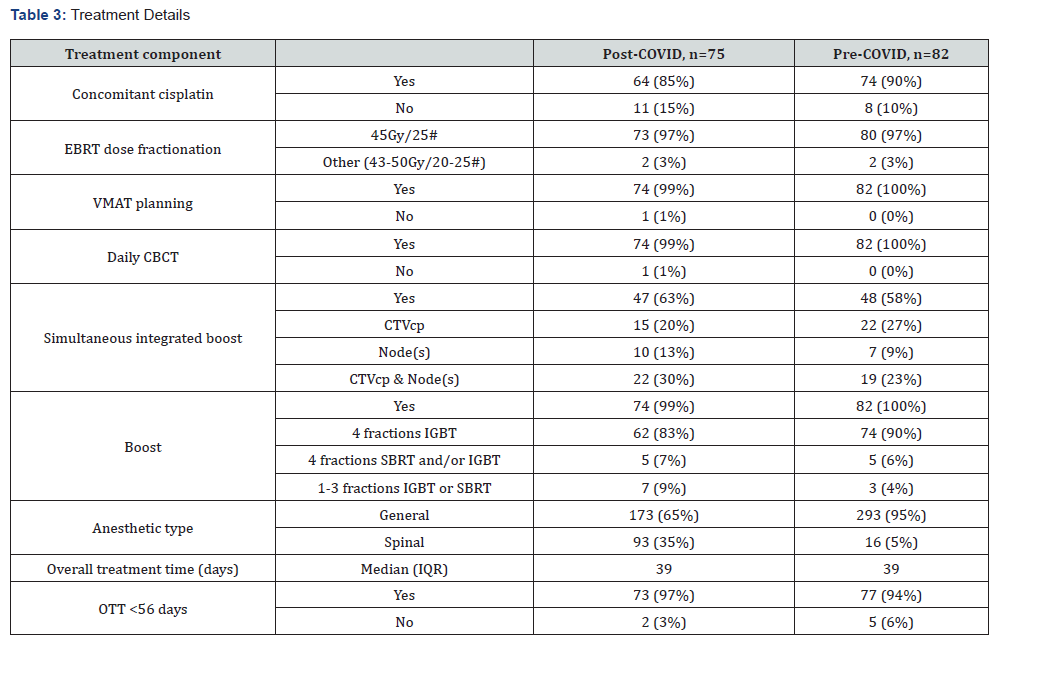
Abbreviations: EBRT: external beam radiotherapy; VMAT: volumetric arc radiotherapy; CBCT: cone beam computed tomography; CTVcp: clinical target volume (central pelvis); IGBT: image guided brachytherapy; IQR: interquartile range; SBRT: stereotactic body radiotherapy; OTT: overall treatment time
EBRT
The prescribed dose fractionation regimen for EBRT was predominantly 45Gy/25# (97% in both cohorts). The proportion of patients completing all EBRT was 90% (74/82) pre-COVID and 99% (74/75) post-COVID. In virtually all cases, EBRT was delivered by VMAT with daily CBCT. SIB was incorporated in approximately 60% of EBRT plans in both cohorts, with a slightly higher frequency of lymph node boosts delivered in the post- COVID period (43% compared with 32% pre-COVID).
IGBT
The majority of patients received four fractions of IGBT over two insertions (90% pre-COVID and 83% post-COVID). A further small proportion received the equivalent of 24Gy/4#, but one or more fractions were delivered with stereotactic body radiotherapy (SBRT). In total, all planned IGBT / SBRT was administered to 96% of patients pre-COVID and 90% post-COVID. The remaining cases had up to three fractions of IGBT and/or SBRT; only one patient did not proceed to a boost as death occurred unexpectedly after one week of EBRT. Prior to the pandemic, the majority of applicator insertions for IGBT were facilitated by general anesthesia, as evidenced by 95% pre-COVID. In contrast, the rate was lower post-COVID at 65%.
OTT
The overall treatment time (OTT) was comparable in both treatment periods. OTT exceeded the UK RCR recommendation of 56 days in less than 5% of patients, predominantly to accommodate urgent SBRT treatment as an alternative to IGBT. There were no gaps in treatment due to COVID-19 infection and no positive PCR tests during CCRT.
Disease control rates
Relapse data and the proportion of patients alive at one- and three-years post diagnosis are depicted in (Table 4); two patients were excluded from the analysis as they were lost to follow up. Persistent disease and/or cumulative relapse rates at one year and three years were 8% and 23% in the pre-COVID cohort and 15% and 29% in the post-COVID cohort, respectively. Local, pelvic, and systemic control rates were all marginally higher in the pre- COVID treatment period. Accordingly, more patients were alive and disease free in the pre-COVID cohort (77%) including four patients who were surgically salvaged (either exenteration for central relapse or resection of a solitary metastasis).
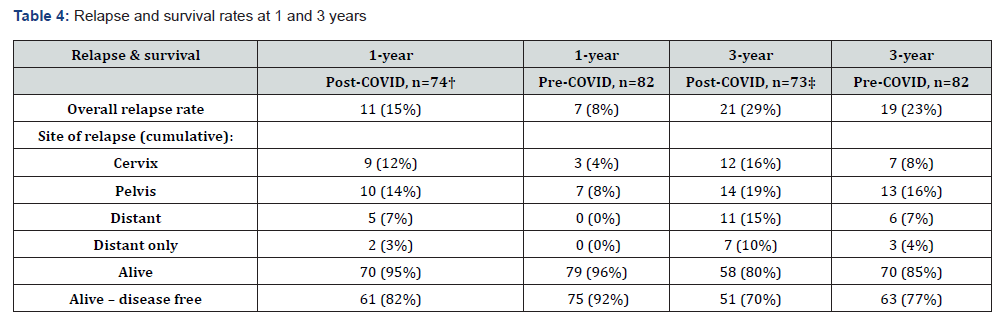
† One patient lost to follow up; two patients lost to follow up.
Discussion
Radiological investigation and clinical management of locally advanced cervical cancer in WOSCAN did not alter significantly in the first 12 months of the COVID-19 pandemic. Deviations from standard CCRT were based largely on medical factors other than intercurrent COVID-19 infection. We observed a higher frequency of FIGO 2018 stage III/IV at presentation post pandemic and a slightly lower proportion of patients were alive and disease free at three years post diagnosis.
Comprehensive cervical cancer control encompasses prevention and screening to detect and treat cervical intraepithelial neoplasia, as well as definitive management of invasive disease. The UK National Health Service (NHS) has a robust screening program; women between the ages of 25 and 49 years are invited for cervical smear every three years, and then 5-yearly until aged 64 years. However, the onset of the COVID-19 pandemic resulted in temporary suspension of screening services. Combined with difficulties in access to primary / secondary care and diagnostics, there were concerns over an initial decline in newly diagnosed cervical cancer cases followed by a potential future rise in locally advanced disease due to missed opportunities to detect and treat high-grade dysplasia and early invasive malignancies. Scottish statistics indicate that cervical cancer incidence reduced by 24% in 2020, with a drop-in rate from 12.3 per 100,000 to 9.4 per 100,000. A similar pattern has emerged in other regions of the UK. For example, a North of England study of six centers indicated a 25.7% reduction in absolute numbers of diagnoses, predominantly accounted for by lower stage cases [10]. Although there are no published reports as yet with estimates of the impact in Scotland beyond 2020, NHS England projected an additional 860 cases of cervical cancer presenting over three years based on a 6-month pause in cervical cancer screening. Likewise, various international reports have described concerning sequelae of compromised screening, including potential adverse effects on survival outcomes [11-15].
We observed a small reduction in the number of referrals for radical CCRT in WOSCAN over the study period, but there was a more notable increased proportion of patients presenting with regional lymph node involvement and/or organ threatening disease at diagnosis. The true impact on stage may have been underestimated as we did not specifically extend the analysis to include patients presenting with widespread IVB disease or locally advanced disease that was deemed too extensive for radical treatment (although it is our practice to offer neoadjuvant chemotherapy to women with very bulky disease in an attempt to downstage [16]). A cancer network in England reported analogous findings; there was no marked difference in tumor size but there was a statistically significant stage migration from FIGO 2018 stage II to III cervical cancer when comparing CCRT cohorts two years post-pandemic with a control group two years prepandemic [17]. Similarly, a US series documented an increase in stage II-IV presentations from 50.1% to 58.8% [18]. Moreover, a Romanian academic center performed a retrospective analysis of patients referred for CCRT and showed that 43.4% presented with FIGO stage III/IV disease in the two years pre-pandemic compared with 59.6% post-pandemic [19]. Conversely, data from a Canadian regional service did not demonstrate any difference on FIGO 2018 stage at diagnosis over the same time period [20]. This variation may be reflective of global patterns in healthcare infrastructure and prioritization.
In order to guide clinicians during an unprecedented time, various national and international guidelines or expert opinions on management of gynecological cancer were rapidly circulated. Much of the focus was on surgical practice, including acceptable delays to definitive surgery. The disruptions to treatment pathways in individual centers or at a population level are now being increasingly described, with a preponderance of reported outcomes in cervical cancer referring to earlier stage disease [21, 22]. In a single systematic review of cervical cancer management, seven of eight published studies indicated that there was a change in treatment delivery and/or treatment delays, although this was often pertaining to localized procedures for high grade dysplasia [23]. In general, hysterectomy rates for early-stage cervical cancer decreased by 15-30% [24]. Several sources suggested that a waiting time of up to six to eight weeks was appropriate for radical hysterectomy in patients with early-stage cervical cancer [25, 26], otherwise CCRT was recommended. As with other centers, theatre availability and critical care capacity was limited in WOSCAN, but the anticipated increase in number of referrals for CCRT in stage I/IIA disease did not occur. Indeed, there were very few patients with operable disease who were directed towards CCRT and the decision was largely based on medical contra-indications to surgery.
Radiotherapy practice in WOSCAN was modified in response to the pandemic. For example, an increased number of patients received radical radiotherapy for esophageal and bladder cancers, whereas cervical cancer statistics remained relatively stable [27]. Overall, there is a paucity of data relating to alteration in CCRT for cervical cancer, but a population-based survey from 14 German university hospitals revealed that weekly performed radiotherapy fractions for cervical cancer decreased by almost 20% in March to August 2020 compared to the control cohort, but this was an inpatient analysis and CCRT is often delivered as an out-patient [28]. No firm explanation was provided for the findings although the authors acknowledge the pattern may reflect reduced diagnoses or altered fractionation. The Romanian study describing stage migration comments that changes to treatment, postponement, and missed days during CCRT all occurred, but does not allude to the specific alterations at an individual patient level. Interestingly, a Turkish survey of gynecological oncologists illustrated a shift towards hypo-fractionated radiotherapy in locally advanced cervical cancer in 57% of respondents, but there was no associated description of precise dose fractionation regimens [29].
UK RCR guidance was to proceed with “lowest number of fractions, typically 45Gy/25#”. The British Gynecological Cancer Society (BGCS), on the other hand, suggested that hypofractionation could be considered but did not recommend any specific regimens [30]. Although some tumor types, particularly breast cancer, rapidly adopted hypo-fractionated radiotherapy during the pandemic, this may have been prompted by randomized evidence produced by the Fast-Forward trial [31]. We did not adopt a hypo-fractionated approach and suspect that many centers were reluctant to increase the fraction size in cervical cancer due to concerns over late effects, despite reports suggesting reasonable pelvic control rates and acceptable toxicity with a total EBRT dose of 39-40Gy utilizing fractions ≥2.5Gy [32].
Our institution has access to a dedicated on-site theatre facility with anesthetic support two days per week. Fortunately, the theatre suite remained functional throughout all waves of the pandemic. As a stand-alone “cold” site with no acute general medical admissions, COVID-19 infections remained low during most of 2020. The only change to our practice was immediately adopting spinal anesthesia, but general anesthesia was phased back in after the first wave. Any delays or alteration in the delivery of brachytherapy were related to non-COVID issues, such as unfavorable anatomy or medical comorbidity. In several cases, we opted to boost the cervix with SBRT. There is a small body of literature on SBRT as a substitute for IGBT [33] and although it should not be considered a routine replacement for IGBT, we have acquired experience in this technique [34]. In other centers, IGBT may have posed more of a problem depending on the infrastructure and provision of theatre services. A US study of the effect of the pandemic on brachytherapy in 47 patients treated February to June 2020 revealed minor treatment delays, but only 15 patients were being treated for cervical cancer [35]. There are several smaller series focused on abbreviated schedules. Rather than follow the EMBRACE protocol of 4 x 7 Gy delivered over one week with two fractions per application which is popular in Europe, regimens such as 3 x 8 - 8.5Gy over one week [36] or even 24 - 48 hours [37, 38] were evaluated, often utilizing a single insertion which minimizes anesthesia and results in a shorter overall treatment time, advantageous from a radiobiological perspective if there had already been a delay due to COVID-19 infection or staffing issues. Preliminary results indicate higher acute mucosal toxicity, but excellent local control rates at 1-2 years; data on late effects are outstanding. It is likely that some centers adopted even more condensed regimens such as 2 x 9 Gy [39, 40]. However, this approach may be associated with compromised local control, especially in the context of large volume residual disease, although this may have been the only option in low resource settings [41].
Disease control rates and survival outcomes were slightly less favorable in the patient group treated post-COVID in our series. We suspect that the reasons for this are multifactorial and reflect the higher proportion of patients presenting with tumors measuring ≥5cm and/or involved nodes, increased frequency of non-squamous pathology, longer time to start treatment, and fewer patients completed all IGBT as planned. Other than the abbreviated schedule IGBT studies already outlined, the only other reported CCRT outcomes after the onset of the pandemic is the Romanian study; patients presenting at advanced stages had a 3.39 higher likelihood of disease progression following CCRT. Further institutional series and population-based studies are awaited.
The major strength of this series is that we have described a real-world depiction of the impact of COVID-19 on the CCRT pathway and outcomes. In addition, the relatively large patient numbers treated at our institution means that we are more likely to detect differences in stage over a one-year period compared with smaller centers. Ideally, Scotland wide data would provide a more robust representation, but we are unable to collect this information by electronic data linkage at present. We acknowledge that as we did not include patients with advanced cervical cancer who received palliative treatment only or best supportive care, we may have underestimated the effect on potential stage migration. Also, it was not always possible to access COVID-19 PCR results that were performed out with routine testing at the central laboratory immediately prior to IGBT, but the clinical notes were reviewed meticulously for any annotations regarding a positive test. Finally, we did not practice high risk CTV outlining as per ESTRO guidelines at this time, as implementing the technique was postponed due to the pandemic, but used central pelvis SIB to boost the cervix.
Conclusions
In summary, although the pressures on the UK NHS in 2020 were immense and the advent of COVID-19 interrupted many aspects of patient care, the investigation and management of locally advanced cervical cancer in WOSCAN did not significantly differ from previous. EBRT practice in particular remained state of the art, but the introduction of MRI based HR-CTV IGBT planning was delayed. The full impact of the pandemic on cervical cancer survival outcomes at an international level are awaited.
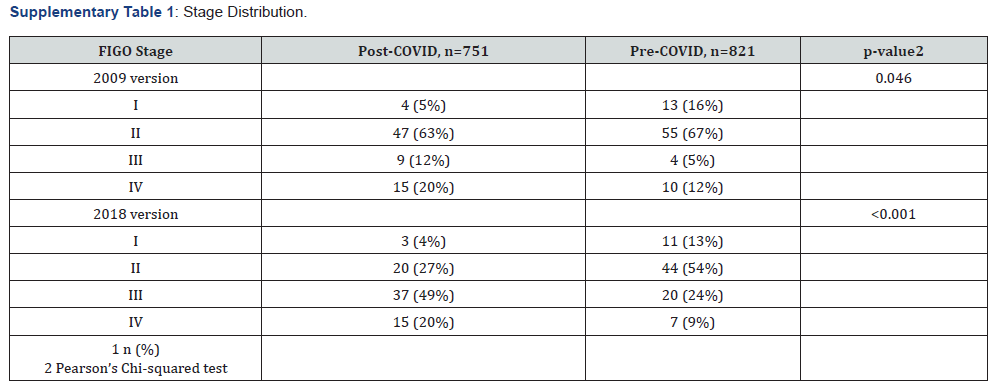
Abbreviations: FIGO – International Federation of Obstetrics & Gynecology
References
- Conibear J, Nossiter J, Foster C, West D, Cromwell D, et al. (2022) The National Lung Cancer Audit: The Impact of COVID-19. Clin Oncol (R Coll Radiol) 34(11): 701-707.
- Nikolopoulos M, Maheshwari MK, Doumouchtsis SK. (2022) Impact of COVID-19 in gynaecological oncology care: a systematic rapid review. Arch Gynecol Obstet 305(3): 555-565.
- Fotopoulou C, Khan T, Bracinik J, Glasbey J, Abu-Rustum N, et al. (2022) Outcomes of gynecologic cancer surgery during the COVID-19 pandemic: an international, multicenter, prospective CovidSurg-Gynecologic Oncology Cancer study. Am J Obstet Gynecol 227(5): 735.e1-735.e25.
- Rose PG, Bundy BN, Watkins EB, Thigpen JT, Deppe G, et al. (1999) Concurrent Cisplatin-Based Radiotherapy and Chemotherapy for Locally Advanced Cervical Cancer. New England Journal of Medicine 340(15): 1144-1153.
- Mei H, Dong X, Wang Y, Tang L, Hu Y (2020) Managing patients with cancer during the COVID-19 pandemic: frontline experience from Wuhan. Lancet Oncol 21(5): 634-636.
- Royal College of Radiologists: gynae-cancer-treatment-covid19.
- Uwins C, Bhandoria GP, Shylasree TS, Butler-Manuel S, Ellis P, et al. (2020) COVID-19 and gynecological cancer: a review of the published guidelines. International Journal of Gynecologic Cancer 30(9): 1424-1433.
- Public Health Scotland: Cancer incidence in Scotland - to December 2020.
- Huang Y, Lu Y, Huang YM, Wang M, Ling W, et al. (2020) Obesity in patients with COVID-19: a systematic review and meta-analysis. Metabolism 113: 154378.
- Davies JM, Spencer A, Macdonald S, Dobson L, Haydock E, et al. (2022) Cervical cancer and COVID-an assessment of the initial effect of the pandemic and subsequent projection of impact for women in England: A cohort study. Bjog 129(7): 1133-1139.
- Burger EA, Jansen EE, Killen J, Kok IM, Smith MA, et al. (2021) Impact of COVID-19-related care disruptions on cervical cancer screening in the United States. J Med Screen 28(2): 213-216.
- Duarte MBO, Argenton JLP, Carvalheira JBC (2022) Impact of COVID-19 in Cervical and Breast Cancer Screening and Systemic Treatment in São Paulo, Brazil: An Interrupted Time Series Analysis. JCO Glob Oncol 8: e2100371.
- Gupta N, Chauhan AS, Prinja S, Pandey AK (2021) Impact of COVID-19 on Outcomes for Patients with Cervical Cancer in India. JCO Glob Oncol 7: 716-725.
- Martins TR, Witkin SS, Mendes-Corrêa MC, Godoy AS, Cury L, et al. (2023) Impact of the COVID-19 Pandemic on Cervical Cancer Screening in São Paulo State, Brazil. Acta Cytol 67(4): 388-394.
- Nickson C, Smith MA, Feletto E, Velentzis LS, Broun K, et al. (2023) A modelled evaluation of the impact of COVID-19 on breast, bowel, and cervical cancer screening programmes in Australia. Elife 12: e82818.
- Baillie K, Reed N, Laskey J, Pan J, Kavanagh K, et al. (2021) Neoadjuvant chemotherapy in locally advanced cervical cancer: real-world data from the Cancer Medicines Outcomes Programme (CMOP) (2023) European Journal of Gynaecological Oncology 42(5): 926-35.
- El-Tawab SS, Fox J, Trent S, Kehoe S (2023) Cervical cancer during the covid pandemic: Are patients presenting with more advanced or larger tumours? Eur J Obstet Gynecol Reprod Biol 283: 95-99.
- Wickenheisser NE, Dillon M, Broadwater G, Zacherl K, Bixel K, et al. (2023) Radical hysterectomy case volume and cervical cancer treatment in the era of COVID-19: A multi-site analysis of National Cancer Institute-designated Comprehensive Cancer Centers. Gynecol Oncol 179: 70-78.
- Popescu A, Pantea S, Radu D, Gluhovschi A, Dumitru C, et al. (2022) The Impact of SARS-CoV-2 Pandemic on Patients Undergoing Radiation Therapy for Advanced Cervical Cancer at a Romanian Academic Center: A Four-Year Retrospective Analysis. Diagnostics (Basel) 12(6): 1488.
- Kerbage Y, Hillmann E, Ruel-Laliberte J, Samouelian V (2025) COVID-19 Pandemic Impact on Delays in Diagnosis and Treatment for Cervical Cancer in Montreal, Canada. Curr Oncol 32(3): 147.
- Lowe-Zinola J, Williamson M, Gaunt E, Boulter H, Pounds R, et al. (2022) Evaluating the impact of the COVID-19 pandemic on tertiary gynaecological cancer care delivery: a population based study. J Obstet Gynaecol 42(8): 3692-3700.
- Manchanda R, Oxley S, Ghaem-Maghami S, Sundar S (2021) COVID-19 and the impact on gynecologic cancer care. Int J Gynaecol Obstet 155 Suppl 1(Suppl 1): 94-101.
- Ferrara P, Dallagiacoma G, Alberti F, Gentile L, Bertuccio P, et al. (2022) Prevention, diagnosis and treatment of cervical cancer: A systematic review of the impact of COVID-19 on patient care. Prev Med 164: 107264.
- Algera MD, van Driel WJ, Slangen BFM, Kruitwagen R, Wouters M (2022) Impact of the COVID-19-pandemic on patients with gynecological malignancies undergoing surgery: A Dutch population-based study using data from the 'Dutch Gynecological Oncology Audit'. Gynecol Oncol 165(2): 330-338.
- Noh KW, Kim B, Choi CH, Kim TJ, Lee JW, et al. (2022) Effect of Waiting Time from Pathological Diagnosis to Definitive Concurrent Chemoradiation for Cervical Cancer on Overall Survival. Cancer Res Treat 54(1): 245-52.
- Matsuo K, Novatt H, Matsuzaki S, Hom MS, Castaneda AV, et al. (2020) Wait-time for hysterectomy and survival of women with early-stage cervical cancer: A clinical implication during the coronavirus pandemic. Gynecol Oncol 158(1): 37-43.
- Grocutt L, Rutherford A, Caldwell D, Wilkinson C, Chalmers AJ, et al. (2023) The Impact of COVID-19 on Radiotherapy Services in Scotland, UK: A Population-based Study. Clinical Oncology 35(2): e227-e234.
- Medenwald D, Brunner T, Christiansen H, Kisser U, Mansoorian S, et al. (2022) Shift of radiotherapy use during the first wave of the COVID-19 pandemic? An analysis of German inpatient data. Strahlentherapie und Onkologie 198(4): 334-345.
- Altın D, Yalçın İ, Khatib G, Dağgez Keleşoğlu M, Akgöl S, et al. (2020) Management of gynecological cancers in the COVID-19 era: a survey from Turkey. J Turk Ger Gynecol Assoc 21(4): 265-271.
- British Gynaecological Cancer Society: BGCS framework for care of patients with gynaecological cancer during the COVID-19 pandemic.
- Brunt AM, Haviland JS, Sydenham M, Agrawal RK, Algurafi H, et al. (2020) Ten-Year Results of FAST: A Randomized Controlled Trial of 5-Fraction Whole-Breast Radiotherapy for Early Breast Cancer. J Clin Oncol 38(28): 3261-3272.
- Mendez LC, Raziee H, Davidson M, Velker V, D'Souza D, et al. (2020) Should we embrace hypofractionated radiotherapy for cervical cancer? A technical note on management during the COVID-19 pandemic. Radiother Oncol 148: 270-273.
- Gultekin M, Yilmaz MT, Yuce Sari S, Yildiz D, Ozyigit G, et al. (2022) Stereotactic body radiotherapy boost in patients with cervical cancer. Journal of Obstetrics and Gynaecology 42(7): 3033-3040.
- Kharul Amali SA, Morris S, Harrand R, Hunter B, Kerr A, et al. (2022) Clinical outcomes of SBRT boost to the cervix as an alternative to intracavitary brachytherapy in locally advanced cervical cancer: Retrospective analysis from the West of Scotland. Int J Gynecol Cancer 32: A23-A24.
- Hathout L, Ennis RD, Mattes MD, Wagman RT, Grann A, et al. (2021) The Impact of COVID-19 on Brachytherapy During the Pandemic: A Rutgers-Robert Wood Johnson Barnabas Health Multisite Experience. Adv Radiat Oncol 6(1): 100600.
- Damast S, Tien CJ, Young M, Altwerger G, Ratner E. (2022) Single application hybrid interstitial brachytherapy for cervical cancer: an institutional approach during the COVID-19 pandemic. J Contemp Brachytherapy 14(1): 66-71.
- Stevens MJ, Ko F, Martland J, Brown R, Bell L, et al. (2023) Safety and efficacy of single insertion accelerated MR-image guided brachytherapy following chemo-radiation in locally advanced cervix cancer: modifying our EMBRACE during the COVID pandemic. Radiat Oncol 18(1): 54.
- Chopra S, Mulani J, Mittal P, Singh M, Shinde A, et al. (2023) Early outcomes of abbreviated multi-fractionated brachytherapy schedule for cervix cancer during COVID-19 pandemic. Brachytherapy 22(2): 125-131.
- Miriyala R, Mahantshetty U (2020) Brachytherapy in cervical cancer radiotherapy during COVID-19 pandemic crisis: problems and prospects. J Contemp Brachytherapy 12(3): 290-293.
- Hendry J, Jones G, Mahantshetty U, Sarria G, da Motta N, et al. (2017) Radiobiological analysis of outcomes using external beam radiotherapy plus high dose-rate brachytherapy (4x7 Gy or 2x9 Gy) for cervical cancer in a multi-institution trial. International Journal of Radiation Oncology, Biology, Physics 99(5): 1313-1314.
- Gangopadhyay A (2022) Elimination of cervical cancer as a public health problem-how shorter brachytherapy could make a difference during COVID-19. Ecancermedicalscience 16: 1352.






























