What is the Molecular Link Between Infertility and Cancer?
Dr Roya Rozati1*, Dr Naila Mohiuddin2, Wajeeda Tabasum2, Taalia Nazeer Ahmed2, Afraa Mohammad2, Dr Vikram Ayapati2, Dr Gautam Ayapati2 and Dr Aleem Ahmed Khan3
1Director, Maternal Health and Research Trust, India
2Research Scholar, Medical Health and Research Institute, India
3CMH Research and Innovation, Bolarum, Secunderabad, India
Submission: July 18, 2023; Published: July 24, 2023
*Corresponding author: Dr Roya Rozati, Director, Maternal Health and Research Trust, India
How to cite this article: Dr Roya R, Dr Naila M, Wajeeda T, Sumaiya N, Taalia Nazeer A, et al.What is the Molecular Link Between Infertility and Cancer?. J Gynecol Women’s Health 2023: 25(3): 556164. DOI: 10.19080/JGWH.2023.25.556164
Abstract
Female unexplained infertility is a multifactorial disorder, and its molecular underpinnings remain poorly understood. Approximately 15-20% of infertility cases are unexplained, posing a significant challenge for reproductive medicine. The endometrial epithelium’s molecular network and signalling pathways play a crucial role in unexplained infertility, where the preservation of structural and functional integrity of maternal uterine tissue is essential for successful implantation and conception. The molecular environment within the uterus influences pregnancy establishment and development, with key components such as extracellular matrix (ECM) components, adhesion molecules, cytokines, and growth factors contributing to structural support and cellular processes. However, the intricate nature of these molecular networks necessitates further investigation. In our study, we employed proteome profiling techniques to analyse uterine tissue samples from women with unexplained infertility, aiming to identify potential alterations in specific proteins and pathways associated with infertility. Interestingly, emerging evidence suggests a molecular link between infertility and cancer in women. Infertility has been associated with an increased risk of certain cancers, including ovarian, endometrial, and breast cancer. Dysregulations in the uterine molecular environment may contribute to the development of cancer by promoting abnormal cell growth, impairing DNA repair mechanisms, and disrupting hormonal balance. Understanding the shared molecular mechanisms between infertility and cancer could provide valuable insights into both conditions. Using high-resolution two-dimensional gel electrophoresis and mass spectrometry, we identified twenty-four differentially expressed proteins, including ANKRD36, ZNF658B, MALRD1, and PRRC2A. Additionally, enrichment analysis revealed numerous pathways associated with infertility morbidity, highlighting their potential relevance to cancer development. These findings underscore the significance of investigating the molecular link between infertility and cancer, paving the way for future research aiming to unravel shared pathways and develop targeted interventions.
Keywords: Female Infertility; Endometrial epithelium; Signalling pathways; Structural integrity; Extracellular matrix (ECM) components; Infertility-associated cancers; Ovarian cancer; Endometrial cancer; Breast cancer
Abbreviations: DEPs: Differentially expressed proteins; 2-DE: Two-dimensional Gel Electrophoresis; MALDI-TOF MS: Matrix-Assisted Laser Desorption/Ionisation Time-Of-Flight Mass Spectrometry; KEGG: Kyoto Encyclopedia of Genes and Genomes; STRING: Search Tool for the Retrieval of Interacting Genes/Proteins; PANTHER: Protein Analysis Through Evolutionary Relationships
Introduction
Approximately 17% of infertility cases remain unexplained without a clearly identified cause [1]. Infertility, a distressing experience particularly affecting women, has been extensively studied in the literature due to its profound impact on various aspects of mental-emotional, social, and cultural well-being [2]. Successful implantation of the fertilised egg, or blastocyst, into the uterine lining is contingent upon a healthy and receptive uterine environment [3]. The integrity of the uterine tissue plays a vital role in facilitating proper attachment and embedding of the blastocyst, establishing crucial connections with the maternal blood supply. Disruptions in maternal uterine integrity, resulting from structural abnormalities, inflammation, or hormonal imbalances, can detrimentally affect the processes of implantation and conception [4].
The intricate nature of embryo implantation and placentation is underscored by the involvement of numerous cytokines and growth factors that exert significant regulatory roles in these processes [5]. Both in mice and humans, deviations from normal expression and functioning of these cytokines and interferons at the tissue level can lead to complete or partial failure of implantation, as well as the occurrence of abnormal placenta formation [6].
Female infertility can be brought about by idiopathic infertility, tubal factor infertility, ovulatory problems, and endometriosis, among other conditions [7]. Approximately 50% of cases of female infertility are attributable to hereditary factors, many of which are yet unknown [8]. Primary infertility refers to females who have never been pregnant, while secondary infertility pertains to those who have had previous pregnancies [9]. In both the cases, gaining a better understanding of the underlying causes and mechanistic pathways is crucial for comprehensive disease comprehension and management.
Intriguingly, emerging evidence suggests a complex molecular link between infertility and cancer in women. Infertility has been associated with an increased risk of certain cancers, including ovarian, endometrial, and breast cancer [10]. Dysregulations in the uterine molecular environment may contribute to the development of cancer by promoting abnormal cell growth, impairing DNA repair mechanisms, and disrupting hormonal balance. Unraveling the shared molecular mechanisms between infertility and cancer holds the potential to provide valuable insights into both conditions, enhancing diagnostic and therapeutic approaches [11].
While microarray studies have identified genomic regions associated with female infertility over the past 20 years, the molecular mechanisms underlying human infertility resulting from these changes remain largely unclear, even in current studies. The advent of high-throughput sequencing technology has facilitated the detection of gene abnormalities related to infertility [12]. Among the omic studies, proteomics has emerged as a valuable tool in the field of human reproduction. Recent advancements in proteomic methods have significantly enhanced the literature on the proteome database, particularly in relation to various reproductive tissues in males and females [13]. Furthermore, network and pathway analysis utilising bioinformatic tools offer a more comprehensive understanding of the potential pathways associated with differentially expressed proteins (DEPs) and their relevance to specific infertility conditions [14].
Proteomics, a study of the temporal and spatial expression of proteins in cells or tissues, enables the assessment of quantitative and qualitative cellular responses related to specific proteins [15,16]. High-resolution two-dimensional gel electrophoresis (2- DE) analysis provides a powerful technique for comparing protein expression patterns, while matrix-assisted laser desorption/ ionisation time-of-flight mass spectrometry (MALDI-TOF MS) aids in identifying protein spots on 2-DE gels [17]. Our study involved the acquisition of paired samples from six endometrial tissue specimens obtained from both infertile and fertile women during the mid-secretory phase (LH +7). These samples underwent two-dimensional gel electrophoresis and mass spectrometry to identify upregulated proteins (Figure 1 provides an overview of our study’s workflow). Subsequently, we examined the physiological pathways associated with the identified proteins to assess the impact of specific genes on the protein expression profile observed in patients with unexplained female infertility and those without infertility.
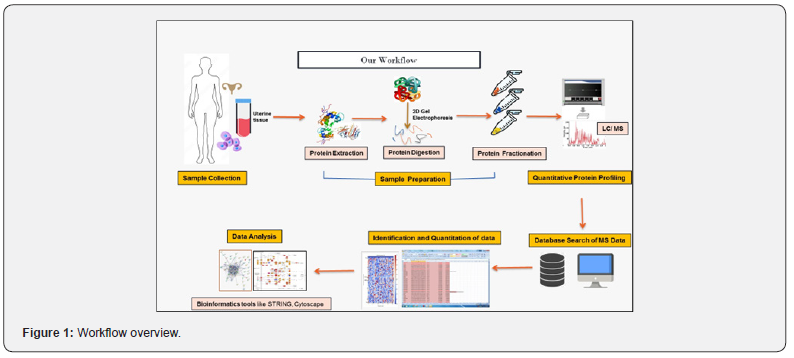
Our goal is to delve into the molecular pathways associated with unexplained infertility through comprehensive analysis of uterine tissue proteomes. By identifying potential alterations and dysregulations in specific proteins and pathways related to this condition, we aim to shed light on the complex molecular networks contributing to its pathogenesis ultimately aiming to reduce embryo wastage and enhance implantation rates. Furthermore, understanding the shared molecular mechanisms between infertility and cancer will provide crucial insights into their interconnected nature, potentially leading to improved strategies for both diagnosis and treatment [18].
Results
Quantitative Protein Profiling Using 2D Gel Electrophoresis and MALDI-TOF
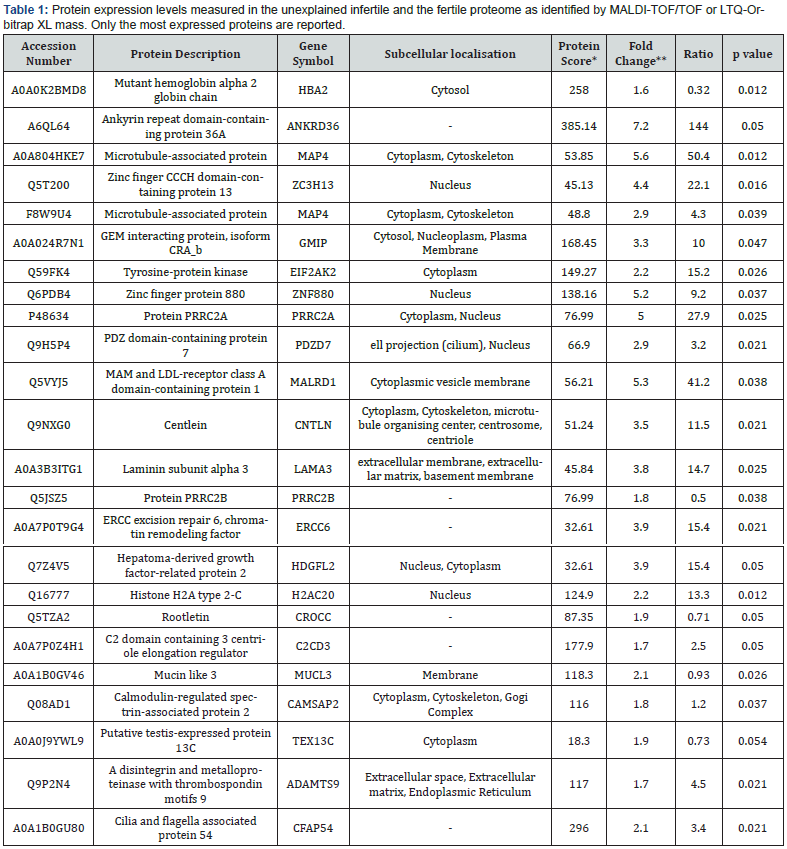
We performed a comparative proteomic analysis of uterine tissues from both groups to generate 2-DE reference maps and identify upregulated proteins. The quantitative analysis utilising Spectrum Mill and Mascot search engines resulted in the identification of over 55 proteins. Among them, 24 proteins exhibited an upregulation of >1.5-fold in cases of unexplained infertility. On average, 1200 spots were detected in the gels for both proteomes. Comparative analysis with normal endometrium revealed a significant elevation of 24 protein locations in the infertile samples (p<0.05). All 24 proteins exhibited a fold change of up to 1.5-fold in terms of expression. Additionally, we identified four down regulated proteins (Haemoglobin beta isoforms), although the differences were not significant and were not further investigated. Protein spots were identified using MALDI-TOF/ TOF and LTQ-Orbitrap XL, with MS/MS data searched against the human section of the UniProt database (Version 20140709, 88,993 sequences) (Table 1).
Bioinformatic Analysis
To elucidate the biological function, pathways, and interaction networks associated with the collected data, we employed various bioinformatic tools and software, including UniProt, Genecards (v.4.8.2), KEGG, Reactome, and STRING (version 10.5). The genes corresponding to differentially expressed proteins were mapped to multiple gene annotation data and biological pathways using PANTHERS 14.0. The categorization of genes was based on molecular function, biological process, route, cellular location, and protein class.
Protein–Protein Interaction and Co-Expression Study
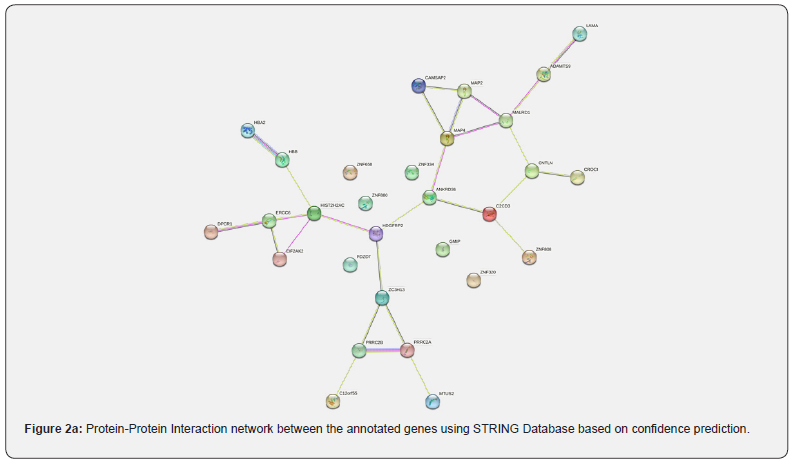
In our study, we utilised the web-based visualisation tool STRING to construct and explore the protein-protein interaction (PPI) network, while Cytoscape (v3.8.2) was employed for additional assessment. The PPI network was represented as an undirected graph, with proteins as nodes and their connections as edges (Figure 2a). Coexpression analysis revealed a strong association among the genes of three metabolic enzymes, including Zinc finger CCCH domain-containing protein 13 and Putative RNA-binding protein 15 (combined score: 0.840), as well as between homologues of Hemoglobin subunit beta- HBB and HBA1 (combined score: 0.781), HBB and HBA2 (combined score: 0.560), and HBA1 and HBA2 (combined score: 0.800). We further analyzed co-expression mapping using the STRING database (Figure 2b) and found the most significant co-expression between PRRC2A and MAP4. Additionally, PRRC2B co-expressed with ERCC6, CNTLN co-expressed with ANKRD36, ZC3H1D co-expressed with EIF2AK2, and CAMSAP2 co-expressed with EIF2AK2. Notably, there is a potential link between EIF2AK2, CAMSAP2, LAMA3, ANKRD36, and unexplained female infertility.

* The protein score is the sum of the Mascot ion scores of all of the peptides that were identified for a given protein; ** the fold change is the ratio of the mean percentage relative volume (%V) (%V = V(single spot)/V(total spot)) of two groups.
Pathway and Functional Enrichment Analysis
We conducted pathway and functional enrichment analysis using the web-based gene set enrichment analysis program EnrichR, which utilises all differentially expressed common genes to identify significant molecular pathways. We employed KEGG, WiKi, and the Reactome pathway databases to analyse the signalling pathways and gene ontology of the differentially expressed genes in our cohort of unexplained female infertility. Signalling pathways and gene ontology (GO) categories, including biological processes, molecular function, and cellular components, were used as annotation sources. Statistical significance was determined based on the adjusted p-value to obtain enhanced results.
Using the PANTHER Database, we characterised the upregulated proteins based on their biological processes, cellular components, and protein classes. We represented the gene ontology of the upregulated proteins using a pie chart (Figure 3a), which revealed that the majority (32.4%) of these genes belong to the category of Basement Membrane, closely followed by the Ribosome category (25.4%). In terms of the influenced biological processes, we observed derangements in microtubule minus-end binding, protein tyrosine kinase activator activity, and kinesin binding activities of the cells (Figure 3b). The majority of the proteins (35.5%) did not fall into any specific Panther class, indicating a lack of research in this area (Figure 3c). Among the known classes, the Gene-specific transcriptional regulator protein class (Panther Category ID: PC00264) exhibited the highest upregulation in our study.
The enrichment study identified 25, 20, and 114 pathways in the KEGG, WiKi, and Reactome databases, respectively. Notably, we considered only the top ten significant pathways from each pathway database. We found several significant pathways, including Proteoglycans in cancer (hsa05205), Notch Signalling (hsa04330), PPAR signalling pathway (hsa03320), Pathways in cancer (hsa05200), Diseases of signal transduction (R-HSA-5663202), CD28 dependent PI3K/Akt signalling (R-HSA-389357), ABC transporters in lipid homeostasis (R-HSA-1369062), VEGFVEGFR2 Signalling Pathway (hsa04370), and Thyroid hormone signalling pathway (hsa04919). These pathways, along with other significant pathways, are depicted in Figure 4a-4c.
Materials and Methods
Patients
We retrospectively recruited twelve infertile women undergoing IVF–ET, hysterectomy, or dilatation and curettage at the Medical Health and Research Institute from February 2021 to February 2023. Informed consent was obtained from all participants after the Institutional Review Board of the study institute authorised the protocol. The study included female subjects under 40 years of age with regular menstrual cycles (27– 35 days), a typical double-phase basal body temperature, normal serum prolactin levels, and a male partner with ≥5 million motile sperms, as recruited in our study.
The cause of infertility was investigated following the minimum propaedeutic procedure for infertile couples. This included hormone and biochemistry profiling, testing for sexually transmitted diseases, imaging examinations, investigation of genetic and/or immunological abnormalities, semen analysis of the partner, hysterosalpingography, hysteroscopy, and laparoscopy (performed in all women up to 36 years old and in patients over 36 years old whenever there were symptoms or abnormalities on imaging examinations). If none of these examinations revealed an abnormality, infertility was considered unexplained. Women who did not achieve pregnancy after at least six natural or induced cycles following laparoscopy were considered infertile. The control group consisted of fertile women with a history of parity and no uterine abnormalities. None of the participants had used any steroidal medications in the past six months. Women with hyperprolactinemia, polycystic ovarian syndrome (PCOS), or moderate-severe endometriosis were excluded. Age, basal serum follicle-stimulating hormone (FSH) levels, and body mass index (BMI) did not significantly differ between the groups. Clinical data and tissue samples were collected after providing a detailed explanation of the study’s objectives. All participants signed an informed consent form approved by the Research Ethics Committee of the Medical Health and Research Institute (MHRI).


Sample Preparation
Biopsies of endometrial tissue were obtained during the mid-secretory phase of the menstrual cycle (LH +7). Endometrial samples were collected under sterile conditions using a pipette catheter and stored at -80 °C for proteome analysis. To deplete serum samples, the ProteoPrep Blue Albumin and IgG Depletion Kit (SIGMA-ALDRICH) was employed, and protein content was measured using the Bradford test. Frozen endometrial tissue samples (40mg) were homogenised using a handheld homogenizer (POLYTRON® System PT 1200E) with 400μl of urea lysis solution (8M urea, 65mM CHAPS, 2 M thiourea, 33 mM Tris, 6 mM PMSF, 65 mM DTT, and 1% protease cocktail inhibitor). The homogenate was then vortexed for 30 seconds at 10-minute intervals (three times) and sonicated on ice using an ultrasonic cleaner in 10-second bursts at 10-second intervals ten times. Subsequently, the sample was centrifuged at 16,000 g for 10 minutes using an Eppendorf centrifuge, and the clear supernatant was collected and stored at -80 °C for further analysis.
DE and Image Analysis
For the first dimension, pH 3-10 13cm IPG strips from GE Healthcare, Uppsala, Sweden, were utilised, and active/passive rehydration was performed. The proteins were focused on an IPGPhor III (GE Healthcare, Uppsala, Sweden) apparatus using the following IEF conditions: 100 V gradient for 1 hour, 300 V gradient for 2 hours, 1000V gradient for 1 hour, 5000V gradient for 5 hours, and 5000 V step, followed by a 5000 V step held for 7 hours at a constant temperature of 20 °C. Each IPG strip underwent isoelectric focusing (IEF), followed by equilibration with 2% DTT and incubation with a different buffer containing 2.5% iodoacetamide in place of DTT. The second dimension PAGE (12.5%) was performed using the SE600 system (GE Healthcare, Uppsala, Sweden) at 1W/gel for 1 hour and 13W/gel for 3 hours. The protein spots were stained with colloidal Coomassie blue G-250 and scanned using a high-precision scanner (ScanMaker 9700XL, Microtek). The gel images were analysed using the gel image analysis tool PDQuest 8.01 (Bio-Rad) [19]. Mass spectrometric analysis was performed on the target protein spots excised from the gel after trypsin digestion. Gel fragments were rinsed with Milli-Q water and treated with a decolorizing solution containing 50% acetonitrile and 25% ammonium bicarbonate. After dehydration in 100% acetonitrile (ACN) for 10 minutes, the decolorized gel particles were vacuum dried for 30 minutes.
Protein Identification and Data Analysis
Protein spots that were excised from the gel were decolorized, digested, and extracted following the protocol described by Liu AX et al. [20]. The peptide samples were analysed using Matrixassisted Laser Desorption Ionization (MALDI-TOF/TOF) Mass Spectrometry and LTQ-Orbitrap XL (Bruker Daltonics, Bremen, Germany). Protein identification was performed using the Bio Tools 3.0 software on MASCOT (V2.1, Matrix Science, UK) based on the peptide mass fingerprint data. Proteins with a MASCOT score greater than 64 and more than four peptide matches were considered significant (P<0.05). Proteins exhibiting a minimum 1.5-fold change between groups and a P-value of 0.05 were subjected to further bioinformatic analysis.
Statistical Analysis
Clinical and experimental data were analysed using Student’s t-tests conducted in SPSS for IBM Statistics version 20 (IBM Corp., Armonk, NY, USA). Only proteins that showed consistent and significant changes (either increased or decreased) were included for subsequent bioinformatics analysis. P-values were calculated using right-tailed Fisher’s exact tests, when appropriate, to identify statistically significant pathways and networks associated with the identified proteins. A significance level of P<0.05 was used for all analyses [21].
Discussion
Infertility, a specific disease entity with a high prevalence, has been considered a social disease [22,23]. While infertility primarily affects reproductive capabilities, it also has far-reaching implications for mental-emotional, social, and cultural well-being. Couples dealing with unexplained infertility often exhibit intrinsic gamete and uterine abnormalities that hinder sperm-oocyte interaction and fertilization, leading to decreased fertility or even complete fertilization failure, even with assisted reproductive techniques [24].
In contrast to males, who generate fresh sperm cells continuously during their lifespan, females possess their complete stock of germ cells at birth, which remains unchanged throughout their lifetime. Many genes, proteins, metabolites, and RNA molecules are all included in the female reproductive interactome, a highly complex network of functionally interconnected biological components. Many diseases are now thought to arise due to biological cascade disruption by changed interactions between different network components.
The endometrial epithelium, which lines the innermost layer of the uterus, plays a pivotal role in establishing a receptive environment for conception. This phase is tightly regulated and occurs approximately 6-10 days after ovulation when the endometrium becomes receptive to embryo attachment and invasion. The uterine luminal epithelium (LE), serving as the first maternal contact for an implanting embryo, undergoes specific changes to facilitate embryo implantation. These changes include intrauterine fluid resorption, cessation of LE proliferation and apoptosis, and conducive LE structural modifications, all of which are essential for establishing transient uterine receptivity for successful implantation [25]. The molecular network and signalling pathways involved in this process are orchestrated by a variety of factors, including hormones, cytokines, growth factors, and cell adhesion molecules.
In contrast to conventional biochemical approaches that monitor only one or a few specific proteins at a time, proteomics is a valuable method for identifying altered protein expression and proteins involved in disease pathogenesis. MS-based proteomics has the potential to detect and quantify any protein within a given sample, making it a more powerful tool compared to microarrays, which are inherently limited to studying the expression of genes with probes spotted on their surface [26]. Therefore, proteomics has emerged as an indispensable tool for evaluating potential pathomechanisms of diseases, both for diagnostic and therapeutic purposes. A recent proteomic analysis of proliferative phase uterine fluid in fertile and infertile women revealed significant differences in secreted proteins, including ECM1 [27]. Although a few studies have investigated the association between genes such as PAI-14G/5G (rs1799889) [28-31] and the risk of infertility, the study of genes associated with infertility in rodent models has expanded the field of translational genetics in identifying the underlying causes of human infertility problems. However, many intriguing aspects of the molecular basis of infertility in humans, including the pathophysiology of unexplained infertility, remain poorly understood even today.
Among the overexpressed proteins in our study, we found few proteins that have been previously described confirming the validity of the quantitative proteomic approach undertaken by us in this study. Among the proteins previously shown to be upregulated in infertility were the Zinc Finger proteins, Claudins and Ankyrin Repeat Domain proteins which were significantly upregulated compared with the endometrial tissue of the control group [31-33]. The cadherin superfamily encompasses an impressive and exhaustive investigation of different categories of molecules engaged in cellular adhesion. These include tight junctional molecules, desmosomal molecules, adhesion molecules from the Ig superfamily, integrins and their ligands, receptors that bind to carbohydrates, galactins, and selectins, which are implicated to also play a role in abnormal cell behaviour leading to development of cancers.
In 1998, Greenhouse et al. embarked on the exploration of the genetic underpinnings of unexplained female infertility, utilising animal models. They hypothesised that abnormalities in the expression or structure of Zfp36l2 could potentially account for approximately 10% of cases of unexplained female infertility in humans [24]. Zfp36l2, also known as zinc finger protein 36-like 2 or TIS11D, ERF2, and BRF2, belongs to a unique family of zinc finger proteins characterised by tandem zinc-binding motifs distinguished by three cysteines followed by one histidine (CCCH) [32]. Zinc finger proteins play pivotal roles in various cellular processes, including gene expression and DNA repair. Concerning female infertility, studies have indicated that specific mutations in zinc finger proteins may impede ovary and follicle development, compromise oocyte quality and viability [33], and disrupt crucial hormonal signalling pathways required for successful reproductive function [34]. In addition to zinc finger proteins, a study by Yu et al. [35] discovered the significance of ankyrin repeat protein ANK6 in fertilisation, particularly in gamete recognition. ANK6, also referred to as At5g61230, exhibited strong expression in both male and female gametophytes before and during fertilisation, but not afterward. Furthermore, a genetic study involving a T-DNA insertional mutant of Ankyrin Repeat proteins demonstrated that alterations in ANK6 expression could result in embryo developmental arrest [36], further emphasising the critical role these proteins play in the infertility process.
Alterations in cadherin expression and function in the uterine endometrium have been implicated in unexplained infertility, affecting cell-cell adhesion and communication. Cadherins like E-cadherin and N-cadherin are crucial for maintaining the structural integrity of the endometrial epithelium and supporting successful embryo implantation. Studies have linked aberrant cadherin expression to impaired embryo attachment and invasion, compromising pregnancy establishment. Notably, E-cadherin dysfunction is associated with endometrial hyperplasia, cancer development, and increased invasion and metastasis of endometrial cancer cells. These findings suggest that cadherin dysfunction may play a role in various endometrial conditions, including infertility. In our study, we observed dysregulation of the cadherin signalling pathway, along with upregulation of other pathways such as Notch Signalling, PPAR signalling pathway, and Pathways in cancer (hsa04330, hsa03320, hsa05200), highlighting their potential relevance. [37-40]. However, it’s important to note that the overall risk of developing cancer as a result of infertility or its treatments remains relatively low, and more research is needed to fully understand the complex relationship between infertility and cancer.
Understanding the potential link between infertility and cancer is crucial for comprehensive disease comprehension and management. Further research is warranted to investigate the underlying molecular mechanisms and identify specific factors and pathways that contribute to both infertility and cancer development. By gaining a better understanding of these shared molecular mechanisms, we can potentially develop more effective strategies for both diagnosing and treating infertility and associated cancers, ultimately improving patient outcomes.
References
- Pisarska MD, Chan JL, Lawrenson K, Gonzalez TL, Wang ET (2019) Genetics and Epigenetics of Infertility and Treatments on Outcomes. J clin Endocrinol Metab104(6): 1871-1886.
- Hasanpoor-Azghdy SB, Simbar M, Vedadhir A (2014) The emotional-psychological consequences of infertility among infertile women seeking treatment: Results of a qualitative study. Iran J Reprod Med 12(2): 131-138.
- Su RW, Fazleabas AT (2015) Implantation and establishment of pregnancy in human and nonhuman primates.
Adv Anat Embryol Cell Biol 216: 189-213. - Simon A, Laufer N (2012) Assessment and treatment of repeated implantation failure (RIF). J Assist Reprod Genet 29(11): 1227-1239.
- Guzeloglu-Kayisli O, Kayisli UA, Taylor HS (2009) The role of growth factors and cytokines during implantation: endocrine and paracrine interactions. Semin Reprod Med 27(1): 62-79.
- Yockey LJ, Iwasaki A (2018) Interferons and Proinflammatory Cytokines in Pregnancy and Fetal Development. Immunity 49(3): 397-412.
- Carson SA, Kallen AN (2021) Diagnosis and Management of Infertility: A Review. JAMA 326(1): 65-76.
- Zorrilla M, Yatsenko AN (2013) The Genetics of Infertility: Current Status of the Field. Curr genet Med Rep 1(4): 10.
- Nachtigall RD (2006) International disparities in access to infertility services. Fertil Steril 85(4): 871-875.
- Cetin I, Cozzi V, Antonazzo P (2008) Infertility as a cancer risk factor - a review. Placenta 29(Suppl B): 169-177.
- Yang Q, Ciebiera M, Bariani MV, Ali M, Elkafas H, et al. (2022) Comprehensive Review of Uterine Fibroids: Developmental Origin, Pathogenesis, and Treatment. Endocr Rev 43(4): 678-719.
- Jiao SY, Yang YH, Chen SR (2021) Molecular genetics of infertility: loss-of-function mutations in humans and corresponding knockout/mutated mice. Hum Reprod Update 27(1): 154-189.
- Kosteria I, Anagnostopoulos AK, Kanaka-Gantenbein C, Chrousos GP, Tsangaris GT, et al. (2017) The Use of Proteomics in Assisted Reproduction. In vivo 31(3): 267-283.
- Proteomics of reproduction: Prospects and perspectives. (2019) Proteomics of Reproduction: Prospects and Perspectives.
Adv Clin Chem 92: 217-243. - Wu YT, Wu Y, Zhang JY, Hou NN, Liu AX, et al. (2015) Preliminary proteomic analysis on the alterations in follicular fluid proteins from women undergoing natural cycles or controlled ovarian hyperstimulation. J Assist Reprod Genet 32(3): 417-427.
- Xiao GG, Recker RR, Deng HW (2008) Recent advances in proteomics and cancer biomarker discovery. Clin Med Oncol 2: 63-72.
- Gharesi-Fard B, Zolghadri J, Kamali-Sarvestani E (2014) Alteration in the expression of proteins in unexplained recurrent pregnancy loss compared with in the normal placenta. J Reprod Dev 60(4): 261-277.
- Lundberg FE, Iliadou AN, Rodriguez-Wallberg K, Gemzell-Danielsson K, Johansson ALV, et al. (2019) The risk of breast and gynecological cancer in women with a diagnosis of infertility: a nationwide population-based study. Eur J Epidemiol 34(5): 499-507.
- https://www.bio-rad.com/en-us/product/pdquest-2-d-analysis-software?ID=966deb78-2656-437f-b7a4-ab0a9bd45c8d
- Liu AX, Jin F, Zhang WW, Zhou TH, Zhou CY, et al. (2006) Proteomic analysis on the alteration of protein expression in the placental villous tissue of early pregnancy loss. Biol Reprod 75(3): 414–420.
- Untergasser A, Cutcutache I, Koressaar T, Ye J, Faircloth BC, et al. (2012) Primer3--new capabilities and interfaces. Nucleic Acids Res 40(15): e115.
- Mascarenhas MN, Flaxman SR, Boerma T, Vanderpoel S, Stevens GA, et al. (2012) National, regional, and global trends in infertility prevalence since 1990: a systematic analysis of 277 health surveys. PLoS Med 9(12): e1001356.
- Boivin J, Bunting L, Collins JA, Nygren KG (2007) International estimates of infertility prevalence and treatment-seeking: potential need and demand for infertility medical care. Human reproduction 22(6): 1506-1512.
- Greenhouse S, Rankin T, Dean J (1998) Genetic causes of female infertility: targeted mutagenesis in mice.
Am J Hum Genet 62(6): 1282-1287. - Ye X (2020) Uterine Luminal Epithelium as the Transient Gateway for Embryo Implantation. Trends Endocrinol Metab 31(2): 165-180.
- Fu X, Fu N, Guo S, et al. (2009) Estimating accuracy of RNA-Seq and microarrays with proteomics. BMC Genomics 10: 161.
- Harriet CF, Jemma E, Nicholas J, Giuseppe I, Andrew WL, et al. (2018) Idiopathic infertility in women is associated with distinct changes in proliferative phase uterine fluid proteins. Biol Reprod 98(6): 752-764.
- Djurovic J, Stojkovic O, Todorovic J, Aleksandra B, Sanja S, et al. (2017) Genetics of suspected thrombophilia in Serbian females with infertility, including three cases, homozygous for FII 20210A or FV 1691A mutations. Hum Fertil 20(2): 132-139.
- Kydonopoulou K, Delkos D, Rousso D (2017) Association of plasminogen activator inhibitor-type 1 (PAI-1) -675 4G/5G polymorphism with unexplained female infertility. Hippokratia 21: 180-185
- Gonçalves-Filho RP, Brandes A, Christofolini DM, Lerner TG, Bianco B, et al. (2011) Plasminogen activator inhibitor-1 4G/5G polymorphism in infertile women with and without endometriosis. Acta Obstet Gynecol Scand 90(5): 473-477.
- Alotaibi FT, Peng B, Klausen C, Lee AF, Abdelkareem AO, et al. (2023) Plasminogen activator inhibitor-1 (PAI-1) expression in endometriosis. Plos One 14(7): e0219064.
- Varnum BC, Ma Q, Chi T, Fletcher B, Herschman HR, et al. (1991) The TIS11 primary response gene is a member of a gene family that encodes proteins with highly conserved sequence containing an unusual cys-his repeats. Mol Cell Biol 11(3): 1754-1758.
- (2018) Chromatin Modification and Global Transcriptional Silencing in the Oocyte Mediated by the mRNA Decay Activator ZFP36L2.
- Gheldof A, Mackay DJG, Cheong Y, Verpoest W (2019) Genetic diagnosis of subfertility: the impact of meiosis and maternal effects. J Med Genet 56(5): 271-282.
- Yu F, Shi J, Zhou J, Gu J, Chen Q, et al. (2010) ANK6, a mitochondrial ankyrin repeat protein, is required for male-female gamete recognition in Arabidopsis thaliana. Proc Natl Acad Sci U S A 107(51): 22332-22337.
- Kulichová K, Pieters J, Kumar V, Honys D, Hafidh S (2022) A Plastid-Bound Ankyrin Repeat Protein Controls Gametophyte and Early Embryo Development in Arabidopsis thaliana. Front Plant Sci 13: 767339.
- Shimono Y, Rikitake Y, Mandai K, Mori M, Takai Y, et al. (2012) Immunoglobulin superfamily receptors and adherens junctions. Sub-cellular biochemistry 60: 137-170.
- Singh H, Aplin JD (2009) Adhesion molecules in endometrial epithelium: tissue integrity and embryo implantation.
J Anat 215(1): 3-13. - Staun-Ram E, Shalev E (2005) Human trophoblast function during the implantation process. Reprod Biol Endocrinol 3: 56.
- Berx G, van Roy F (2009) Involvement of members of the cadherin superfamily in cancer. Cold Spring Harb Perspect Biol 1(6): a003129.






























