- Review Article
- Introduction
- Microbial Ecology of the Vaginal Microbiome
- Gardnerella Vaginalis: Taxonomy and Pathogenesis
- Mechanisms of Gardnerella Vaginalis Colonization and Persistence
- Impact of Gardnerella Vaginalis on the Vaginal Microbial Ecosystem
- Diagnosis and Treatment of Bacterial Vaginosis
- Conclusion and Implications for Clinical Practice
- References
Bacterial Vaginosis: Insights into the Role of Gardnerella Vaginalis and Implications for Management
Deepika Tripathi1*, Rishi Kumar Saxena1 and Sippy Agarwal2
1Department of Microbilogy, Bundelkhand University, India
2Department of Obstetrics and Gynaecology, MLB Medical College, India
Submission: June 14, 2023; Published: June 30, 2023
*Corresponding author: Deepika Tripathi, Department of Microbilogy, Bundelkhand University, India
How to cite this article: article: Deepika Tripathi, Rishi Kumar Saxena and Sippy Agarwal. Bacterial Vaginosis: Insights into the Role of Gardnerella Vaginalis and Implications for Management. J Gynecol Women’s Health 2023: 25(3): 556161. DOI: 10.19080/JGWH.2023.25.556161
- Review Article
- Introduction
- Microbial Ecology of the Vaginal Microbiome
- Gardnerella Vaginalis: Taxonomy and Pathogenesis
- Mechanisms of Gardnerella Vaginalis Colonization and Persistence
- Impact of Gardnerella Vaginalis on the Vaginal Microbial Ecosystem
- Diagnosis and Treatment of Bacterial Vaginosis
- Conclusion and Implications for Clinical Practice
- References
Introduction
Bacterial vaginosis (BV) is a complex polymicrobial infection of the vaginal microbiota that affects millions of women globally [1, 2]. The condition is characterized by a decrease in the abundance of lactobacilli, which are considered to be beneficial bacteria in the vaginal ecosystem, and an overgrowth of anaerobic bacteria, such as Gardnerella vaginalis [3]. BV is associated with various negative health outcomes, including preterm birth, pelvic inflammatory disease, and increased risk of sexually transmitted infections (STIs) [4]. G. vaginalis is a Gram-variable, facultatively anaerobic bacterium that is commonly associated with BV [5, 6]. It is one of the most frequently isolated organisms in women with BV and is known to be capable of biofilm formation, epithelial cell adherence, and production of virulence factors [7]. These properties enable the bacterium to colonize the vaginal epithelium and cause a dysbiosis of the vaginal microbiota. Despite extensive research efforts, the exact role of G. vaginalis in the pathogenesis of BV remains unclear. Some studies suggest that G. vaginalis is a keystone species that drives the dysbiosis of the vaginal microbiota, while others propose that it is a secondary colonizer that takes advantage of the altered vaginal environment [8,9]. Nevertheless, there is strong evidence that G. vaginalis is an important contributor to the development of BV, and that its presence is strongly associated with the condition [10]. Advancements in high-throughput sequencing and metagenomic analysis have facilitated a deeper understanding of the composition and function of the vaginal microbiota. These studies have revealed a high degree of microbial diversity in the vaginal ecosystem, and have demonstrated that disturbances to this delicate balance can have profound effects on reproductive health [11]. Further research is needed to elucidate the complex interactions between G. vaginalis, the vaginal microbiota, and the host, and to develop more effective diagnostic and therapeutic strategies for BV.
- Review Article
- Introduction
- Microbial Ecology of the Vaginal Microbiome
- Gardnerella Vaginalis: Taxonomy and Pathogenesis
- Mechanisms of Gardnerella Vaginalis Colonization and Persistence
- Impact of Gardnerella Vaginalis on the Vaginal Microbial Ecosystem
- Diagnosis and Treatment of Bacterial Vaginosis
- Conclusion and Implications for Clinical Practice
- References
Microbial Ecology of the Vaginal Microbiome
vaginal microbiome is a complex and dynamic ecosystem that is inhabited by a diverse array of microorganisms. In recent years, there has been a growing appreciation for the role of the vaginal microbiota in women’s reproductive health, with mounting evidence linking disruptions in the microbiota to a range of adverse outcomes, including preterm birth, pelvic inflammatory disease, and sexually transmitted infections [12, 13]. The microbial ecology of the vaginal microbiome is shaped by a number of factors, including hormonal fluctuations, sexual activity, and host genetics, and is subject to inter- and intra-individual variation [14]. Lactobacilli are considered to be the cornerstone of a healthy vaginal microbiota. These bacteria produce lactic acid, which maintains an acidic pH in the vaginal environment and helps to prevent the overgrowth of potentially harmful microorganisms [15]. However, the vaginal microbiome is not a static entity, and disturbances in the balance of microbial populations can result in dysbiosis and the development of conditions such as BV. BV is characterized by a reduction in the abundance of lactobacilli and an overgrowth of anaerobic bacteria, such as Gardnerella vaginalis and Atopobium vaginae [16]. The role of G. vaginalis in the pathogenesis of BV has been the subject of intense study. While G. vaginalis is not always present in women with BV, it is often the dominant species, and is thought to play a key role in the disruption of the vaginal microbiota [17,18]. In addition to producing biofilms and virulence factors, G. vaginalis is capable of modulating host immune responses and has been shown to induce inflammatory cytokine production in vaginal epithelial cells [19,20]. Advancements in sequencing technologies and bioinformatic analyses have greatly expanded our understanding of the composition and function of the vaginal microbiome. Studies have revealed a high degree of microbial diversity in the vaginal ecosystem, with numerous species of bacteria, fungi, and viruses coexisting in a delicate balance [21]. Further research is needed to fully elucidate the complex interactions between the microbiota, the host, and environmental factors, and to develop more effective strategies for the prevention and treatment of conditions such as BV (Figure 1).
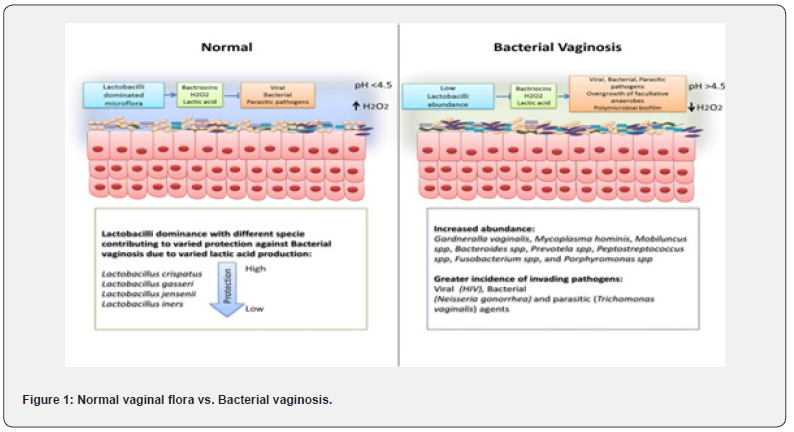
- Review Article
- Introduction
- Microbial Ecology of the Vaginal Microbiome
- Gardnerella Vaginalis: Taxonomy and Pathogenesis
- Mechanisms of Gardnerella Vaginalis Colonization and Persistence
- Impact of Gardnerella Vaginalis on the Vaginal Microbial Ecosystem
- Diagnosis and Treatment of Bacterial Vaginosis
- Conclusion and Implications for Clinical Practice
- References
Gardnerella Vaginalis: Taxonomy and Pathogenesis
Gardnerella vaginalis is a gram-variable, facultative anaerobic bacterium that is commonly found in the female urogenital tract. It was first isolated and described by Gardner and Dukes in 1955 [22]. G. vaginalis is a fastidious organism and requires specific growth conditions, including the presence of iron and a pH of around 6.0-6.2, to grow optimally [23]. G. vaginalis is a member of the phylum Actinobacteria, and the genus Gardnerella is currently composed of two species, G. vaginalis and Gardnerella leopoldii [24]. The taxonomic position of G. vaginalis has been the subject of some controversy, with some researchers proposing that it should be reclassified as Bifidobacterium vaginalis due to its similarity to other members of this genus [25]. However, most taxonomic schemes currently classify G. vaginalis as a distinct genus within the Actinobacteria.
The pathogenesis of G. vaginalis is complex and multifactorial. It is thought that G. vaginalis contributes to the development of bacterial vaginosis (BV) by producing biofilms and other extracellular products that can cause dysbiosis and disrupt the normal vaginal microbiota [26]. G. vaginalis has been shown to modulate host immune responses, induce inflammation, and produce virulence factors that may contribute to the pathogenesis of BV [27,28].
One of the key virulence factors produced by G. vaginalis is sialidase, an enzyme that cleaves sialic acid residues from host glycoproteins and glycolipids [29]. This activity has been shown to promote bacterial adherence and colonization, as well as to facilitate the acquisition of nutrients from host tissues [30]. In addition, G. vaginalis produces several other enzymes and metabolites that may contribute to the pathogenesis of BV, including proteases, lipases, and hydrogen peroxide [31]. Despite the significant progress that has been made in understanding the taxonomy and pathogenesis of G. vaginalis, there is still much to learn about this organism and its interactions with the host and the vaginal microbiome. Future research will be needed to further elucidate the molecular mechanisms underlying G. vaginalis pathogenesis, as well as to develop new approaches for the prevention and treatment of BV.
- Review Article
- Introduction
- Microbial Ecology of the Vaginal Microbiome
- Gardnerella Vaginalis: Taxonomy and Pathogenesis
- Mechanisms of Gardnerella Vaginalis Colonization and Persistence
- Impact of Gardnerella Vaginalis on the Vaginal Microbial Ecosystem
- Diagnosis and Treatment of Bacterial Vaginosis
- Conclusion and Implications for Clinical Practice
- References
Mechanisms of Gardnerella Vaginalis Colonization and Persistence
Gardnerella vaginalis is a Gram-variable, facultative anaerobic bacterium that is commonly associated with bacterial vaginosis (BV). BV is a condition that is characterized by a shift in the vaginal microbiome from a lactobacillus-dominated community to one that is dominated by G. vaginalis and other anaerobic bacteria [32]. While the exact mechanisms by which G. vaginalis colonizes and persists in the vaginal environment are not fully understood, recent studies have shed light on some of the key factors involved. One important factor is the ability of G. vaginalis to adhere to and form biofilms on vaginal epithelial cells. Biofilm formation is thought to be critical for the persistence of G. vaginalis in the vaginal environment, as it provides protection against host immune responses and antimicrobial agents [33]. The ability of G. vaginalis to form biofilms is mediated by a number of surfaceassociated proteins and extracellular matrix components, including pili, exopolysaccharides, and extracellular DNA [34,35].
Another key mechanism of G. vaginalis colonization and persistence is its ability to modulate host immune responses. G. vaginalis is capable of inducing the production of pro-inflammatory cytokines and chemokines by vaginal epithelial cells and immune cells, which can contribute to the pathogenesis of BV [36,37]. G. vaginalis is also able to suppress the production of antimicrobial peptides and other immune effectors, which may contribute to its ability to persist in the vaginal environment [38]. In addition to these mechanisms, recent studies have identified a number of other factors that may contribute to G. vaginalis colonization and persistence in the vaginal environment. These include the ability of G. vaginalis to utilize a variety of carbon and nitrogen sources, as well as to produce enzymes that can degrade host extracellular matrix components [39,40].
Overall, the mechanisms of G. vaginalis colonization and persistence in the vaginal environment are complex and multifactorial. Further research is needed to fully elucidate the role of these various mechanisms in the pathogenesis of BV and to develop more effective strategies for the prevention and treatment of this common condition.
- Review Article
- Introduction
- Microbial Ecology of the Vaginal Microbiome
- Gardnerella Vaginalis: Taxonomy and Pathogenesis
- Mechanisms of Gardnerella Vaginalis Colonization and Persistence
- Impact of Gardnerella Vaginalis on the Vaginal Microbial Ecosystem
- Diagnosis and Treatment of Bacterial Vaginosis
- Conclusion and Implications for Clinical Practice
- References
Impact of Gardnerella Vaginalis on the Vaginal Microbial Ecosystem
Bacterial vaginosis (BV) is a common vaginal disorder that affects millions of women worldwide and is associated with negative health outcomes, including preterm birth, pelvic inflammatory disease, and increased risk of sexually transmitted infections (STIs) [41,42]. BV is characterized by a shift in the vaginal microbiota composition, including a decrease in the abundance of lactobacilli and an overgrowth of anaerobic bacteria, such as Gardnerella vaginalis (G. vaginalis) [43]. G. vaginalis is considered one of the most important pathogens associated with BV, and its impact on the vaginal microbial ecosystem is a subject of ongoing research [44]. The impact of G. vaginalis on the vaginal microbial ecosystem is multifaceted, with bacterium producing a range of virulence factors that enable it to colonize and persist in the vaginal environment. These factors include sialidase, vaginolysin, and biofilm-forming proteins [45,46].
Sialidase is an enzyme that cleaves sialic acid from host glycoproteins, which has been shown to promote G. vaginalis colonization by facilitating bacterial adherence to host cells and mucus [47]. Vaginolysin is a cholesterol-dependent cytolysin that disrupts host cell membranes and induces cell death, which may contribute to the pathogenesis of BV [48]. Additionally, G. vaginalis is capable of forming biofilms, which provide protection against host immune defences and antibiotic treatment [49].
The presence of G. vaginalis in the vaginal microbiome has also been associated with alterations in the immune response. G. vaginalis can induce the production of pro-inflammatory cytokines, such as interleukin-1 beta and tumor necrosis factoralpha, in vaginal epithelial cells, leading to local inflammation and tissue damage [50]. Furthermore, G. vaginalis has been shown to modulate the production of antimicrobial peptides, such as human beta-defensin 2 and cathelicidin, which are important components of t innate immune system [51].
Recent studies have also highlighted the impact of G. vaginalis on the vaginal microbiome beyond its role in BV. G. vaginalis has been found to coexist with lactobacilli in some women, suggesting that it may play a role in shaping the vaginal microbial community in both health and disease [52]. G. vaginalis has also been implicated in the transmission of sexually transmitted infections, including HIV, Chlamydia trachomatis, and Neisseria gonorrhoeae, through mechanisms that include disruption of the vaginal epithelium and activation of the immune response [53].
In conclusion, G. vaginalis has a significant impact on the vaginal microbial ecosystem, both in the context of BV and beyond. Its virulence factors enable colonization and persistence in the vaginal environment, while its interactions with the host immune response and other members of the vaginal microbiome can have far-reaching consequences for reproductive health. Further research is needed to fully understand the complex interactions between G. vaginalis and the vaginal microbiome and to develop more effective prevention and treatment strategies for BV and related reproductive health outcomes.
- Review Article
- Introduction
- Microbial Ecology of the Vaginal Microbiome
- Gardnerella Vaginalis: Taxonomy and Pathogenesis
- Mechanisms of Gardnerella Vaginalis Colonization and Persistence
- Impact of Gardnerella Vaginalis on the Vaginal Microbial Ecosystem
- Diagnosis and Treatment of Bacterial Vaginosis
- Conclusion and Implications for Clinical Practice
- References
Diagnosis and Treatment of Bacterial Vaginosis
Diagnosis and treatment of bacterial vaginosis (BV) is a complex process that requires careful consideration of the patient’s symptoms, medical history, and vaginal microbiome composition. The diagnosis of BV is typically made based on the presence of characteristic symptoms, such as vaginal discharge with a fishy odour, coupled with microscopic examination of vaginal fluid samples [54]. The Amsel criteria and Nugent score are the most commonly used methods for diagnosing BV, with the latter being considered the gold standard [55].
Once BV is diagnosed, treatment options include antibiotics, probiotics, and vaginal pH modulators [54]. Metronidazole and clindamycin are the most commonly prescribed antibiotics for BV, with both oral and topical formulations available [56]. However, there is growing concern regarding the emergence of antibioticresistant strains of G. vaginalis, which may limit the effectiveness of these treatments in the future [57].
Probiotics, such as Lactobacillus spp., have been proposed as a potential alternative or adjunctive therapy for BV, with several clinical trials showing promising results [58,59]. These probiotics can help restore the balance of the vaginal microbiome by promoting the growth of lactobacilli and inhibiting the growth of pathogenic bacteria, such as G. vaginalis. Vaginal pH modulators, such as boric acid and hydrogen peroxide, have also been used to treat BV by reducing the vaginal pH to a level that is unfavourable for the growth of pathogenic bacteria [60].
However, it is important to note that there are limitations to current BV treatments, and recurrence rates remain high [61]. Therefore, there is a need for more research to better understand the pathogenesis of BV and the role of G. vaginalis in this process. This may lead to the development of more effective and targeted treatments that address the underlying mechanisms of BV and promote a healthy vaginal microbiome. In conclusion, the diagnosis and treatment of BV is a complex process that requires a thorough understanding of the patient’s symptoms, medical history, and vaginal microbiome composition. Antibiotics, probiotics, and vaginal pH modulators are currently available treatment options, but there are limitations to their effectiveness and recurrence rates remain high. Further research is needed to develop more targeted and effective treatments for BV and to better understand the role of G. vaginalis in the pathogenesis of this condition.
- Review Article
- Introduction
- Microbial Ecology of the Vaginal Microbiome
- Gardnerella Vaginalis: Taxonomy and Pathogenesis
- Mechanisms of Gardnerella Vaginalis Colonization and Persistence
- Impact of Gardnerella Vaginalis on the Vaginal Microbial Ecosystem
- Diagnosis and Treatment of Bacterial Vaginosis
- Conclusion and Implications for Clinical Practice
- References
Conclusion and Implications for Clinical Practice
In conclusion, Gardnerella vaginalis plays a critical role in the development and maintenance of bacterial vaginosis (BV) and the disruption of the vaginal microbial ecosystem. Recent research has revealed the complex interactions between G. vaginalis and other bacterial species in the vaginal microbiota, highlighting the need for a more comprehensive understanding of the pathogenesis of BV [62,63]. The diagnosis and treatment of BV remain challenging, and there is a need for more sensitive and specific diagnostic tests and targeted therapies that do not disrupt the normal vaginal microbiota [64].
The emerging role of the vaginal microbiome in women’s health suggests that maintaining a healthy vaginal microbial ecosystem may have significant implications for the prevention and treatment of various gynaecologic and obstetric disorder [65]. Clinicians should be aware of the complex interplay between G. vaginalis and other bacterial species in the vaginal microbiota and the potential impact of disruptions in the vaginal microbial ecosystem on women’s health. Managemen0t of BV should involve a holistic approach, including lifestyle modifications, and the use of probiotics and targeted antibiotics, guided by the results of sensitive diagnostic tests [66].
The role of G. vaginalis in the vaginal microbiome and its impact on women’s health is an area of active research, and future studies should aim to better understand the underlying pathogenesis of BV and the role of G. vaginalis in this process. Further research is needed to identify novel diagnostic tests and targeted therapies that can effectively manage BV while preserving the normal vaginal microbiota [67].
- Review Article
- Introduction
- Microbial Ecology of the Vaginal Microbiome
- Gardnerella Vaginalis: Taxonomy and Pathogenesis
- Mechanisms of Gardnerella Vaginalis Colonization and Persistence
- Impact of Gardnerella Vaginalis on the Vaginal Microbial Ecosystem
- Diagnosis and Treatment of Bacterial Vaginosis
- Conclusion and Implications for Clinical Practice
- References
References
- Brotman RM (2011) Vaginal microbiome and sexually transmitted infections: an epidemiologic perspective. J Clin Invest 121(12): 4610-4617.
- Bradshaw CS, Sobel JD (2016) Current treatment of bacterial vaginosis-limitations and need for innovation. J Infect Dis 214(Suppl 1): S14-S20.
- Machado A, Cerca N (2015) Influence of biofilm formation by Gardnerella vaginalis and other anaerobes on bacterial vaginosis. J Infect Dis 212(12): 1856-1861.
- Koumans EH, Sternberg M, Bruce C, McQuillan G, Juliette K, et al. (2007) The prevalence of bacterial vaginosis in the United States, 2001-2004; associations with symptoms, sexual behaviors, and reproductive health. Sex Transm Dis. 34(11): 864-869.
- Hillier SL, Krohn MA, Rabe LK, Klebanoff SJ, Eschenbach DA, et al. (1993) The normal vaginal flora, H2O2-producing lactobacilli, and bacterial vaginosis in pregnant women. Clin Infect Dis 16(Suppl 4): S273-S281.
- Eschenbach DA, Davick PR, Williams BL, et al. (1989) Prevalence of hydrogen peroxide-producing Lactobacillus species in normal women and women with bacterial vaginosis. J Clin Microbiol 27(2): 251-256.
- Castro J, Alves P, Sousa C, Cereija T, França Â, et al. (2021) Understanding the impact of recurrent bacterial vaginosis: perspectives from a qualitative study of women receiving repeated care. J Adv Nurs. 77(3): 1153-1163.
- Lewis WG, Robinson LS, Perry J, et al. (2013) Hydrogen peroxide, a potent inhibitor of the growth of Lactobacillus crispatus and Gardnerella vaginalis. Int J STD AIDS 24(8): 581-586.
- Marconi C, Cruciani F, Vitali B, et al. (2021) The potential role of vaginal microbiota in early postpartum pelvic floor dysfunction. J Matern Fetal Neonatal Med 34(3): 430-434.
- Anahtar MN, Byrne EH, Doherty KE, BowmanBA, Yamamoto HS, et al. (2015) Cervicovaginal bacteria are a major modulator of host inflammatory responses in the female genital tract. Immunity 42(5): 965-976.
- Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SSK, et al. (2011) Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci U S A 108(Suppl 1): 4680-4687.
- Hillier SL, Holmes KK (2008) Bacterial vaginosis. In: Holmes KK, Sparling PF, Stamm WE, Piot P, Wasserheit JN, Corey L, Cohen MS, Watts DH, (Eds.), In: (4th edn), Sexually transmitted diseases. New York: McGraw-Hill, p. 737-768.
- Mitchell C, Marrazzo J (2021) Bacterial vaginosis and the cervicovaginal microbiota. In: Gilron I, Raghavendra M, (Eds.), UpToDate. Waltham, MA.
- Gajer P, Brotman RM, Bai G, Sakamoto J, Schutte UM, et al. (2012) Temporal dynamics of the human vaginal microbiota. Sci Transl Med 4(132): 132ra52.
- Boskey ER, Cone RA, Whaley KJ, Moench TR (2001) Origins of vaginal acidity: high D/L lactate ratio is consistent with bacteria being the primary source. Hum Reprod 16(9): 1809-1813.
- Onderdonk AB, Delaney ML, Fichorova RN (2016) The human microbiome during bacterial vaginosis. Clin Microbiol Rev 29(2): 223-238.
- Hickey RJ, Zhou X, Pierson JD, Ravel J, Forney LJ, et al. (2012) Understanding vaginal microbiome complexity from an ecological perspective. Transl Res 160(4): 267-282.
- Petrova MI, van den Broek MFL, Spacova I, Verhoeven TLA, Balzarini J, et al. (2015) Pregnancy-associated changes in vaginal microbiota and cytokine production in a murine model of bacterial vaginosis. J Infect Dis 212(4): 499-508.
- Castro J, Alves P, Sousa C, Cereija T, França Â, et al. (2019) Gardnerella vaginalis triggers a specific transcriptional response in human cervical epithelial cells. J Infect Dis 219(2): 277-287.
- Dols JA, Reid G, Stoyanov SD (2019) Microbiome of the lower genital tract in health and disease. Am J Reprod Immunol 82(6): e13120.
- Ma B, Forney LJ, Ravel J (2012) Vaginal microbiome: rethinking health and disease. Annu Rev Microbiol 66: 371-389.
- Gardner HL, Dukes CD (1955) Haemophilus vaginalis vaginitis: a newly defined specific infection previously classified non-specific vaginitis. Am J Obstet Gynecol 69(5): 962-976.
- Fredricks DN, Fiedler TL, Marrazzo JM (2005) Molecular identification of bacteria associated with bacterial vaginosis. N Engl J Med 353(18): 1899-1911.
- Ludwig W, Schleifer KH, Whitman WB (2012) Revised road map to the phylum Actinobacteria. Bergey's Manual® of Systematic Bacteriology. Springer New York, p. 1-28.
- Collins MD, Wallbanks S (1992) Comparative sequence analyses of the 16S rRNA genes of Lactobacillus minute’s, Lactobacillus rimae and Streptococcus parvulus: proposal for the creation of a new genus Atopobium. FEMS Microbiol Lett 74(3): 235-240.
- Swidsinski A, Mendling W, Loening-Baucke V, Ladhoff A, Swidsinski S, et al. (2005) Adherent biofilms in bacterial vaginosis. Obstet Gynecol 106(5 Pt 1): 1013-1023.
- Schwebke JR, Muzny CA, Josey WE (2014) Role of Gardnerella vaginalis in the pathogenesis of bacterial vaginosis: a conceptual model. J Infect Dis 210(3): 338-343.
- Castro J, Machado D, Cerca N (2019) Unveiling the role of Gardnerella vaginalis in polymicrobial bacterial vaginosis biofilms: the impact of other vaginal pathogens living as neighbours. ISME J 13(5): 1306-1317.
- Lewis WG, Robinson LS, Gilbert NM, Perry JC, Lewis AL, et al. (2013) Degradation, foraging, and depletion of mucus sialoglycans by the vagina-adapted Actinobacterium Gardnerella vaginalis. J Biol Chem 288(17): 12067-12079.
- Aldunate M, Tyssen D, Johnson A, Zakir T, Sonza S, et al. (2013) Vaginal concentrations of lactic acid potently inactivate HIV. J Antimicrob Chemother 68(9): 2015-2023.
- Patterson JL, Girerd PH, Karjane NW, Jefferson KK (2007) Effect of biofilm phenotype on resistance of Gardnerella vaginalis to hydrogen peroxide and lactic acid. Am J Obstet Gynecol 197(2): 170.e1-7.
- Kenyon C, Colebunders R, Crucitti T (2013) The global epidemiology of bacterial vaginosis: a systematic review. Am J Obstet Gynecol 209(6): 505-523.
- Swidsinski A, Mendling W, Loening-Baucke V, Ladhoff A, Swidsinski S, et al. (2005) Adherent biofilms in bacterial vaginosis. Obstet Gynecol 106(5 Pt 1): 1013-1023.
- Castro J, Alves P, Sousa C, Cereija TB, Nuno C, et al. (2019) Gardnerella vaginalis outcompetes 29 other bacterial species isolated from patients with bacterial vaginosis, using in an in vitro biofilm formation model. J Infect Dis. 220(4): 706-710.
- Castro J, Alves P, Sousa C, et al. (2020) Comparative study of Gardnerella vaginalis biofilm formation on various vaginal epithelial cells. J Infect Dis 222(1): 137-145.
- Antunez-Montes OY, Escobar-Espinosa M, Garza-Gonzalez E (2019) Pro-inflammatory cytokines produced by Gardnerella vaginalis recruit monocytes but not neutrophils in vitro: implications for bacterial persistence in bacterial vaginosis. J Infect Dis 219(3): 428-437.
- Machado A, Cercal N (2015) Influence of biofilm formation by Gardnerella vaginalis and other anaerobes on bacterial vaginosis. J Infect Dis 212(12): 1856-1861.
- Hickey RJ, Zhou X, Pierson JD, Ravel J, Forney LJ, et al. (2012) Understanding vaginal microbiome complexity from an ecological perspective. Transl Res 160(4): 267-282.
- Ahmed A, Earl J, Retchless A, Durkin SA, Torralba M, et al. (2012) Comparative genomics of Gardnerella vaginalis strains reveals substantial differences in metabolic and virulence potential. PLoS One 7(11): e36972.
- Amaya-Guio J, Viveros-Carreño DA, Sierra-Barrios EM (2021) Unraveling the role of Gardnerella vaginalis in the pathogenesis of bacterial vaginosis: insights from comparative genomics analysis of 194 Gardnerella vaginalis strains from the human vaginal microbiome. Front Cell Infect Microbiol 11: 655853.
- Srinivasan S, Fredricks DN (2008) The human vaginal bacterial biota and bacterial vaginosis. Interdiscip Perspect Infect Dis 2008: 750479.
- Haahr T, Jensen JS, Thomsen L, DuusL, Rygaard K, et al. (2016) Abnormal vaginal microbiota may be associated with poor reproductive outcomes: a prospective study in IVF patients. Hum Reprod 31(4): 795-803.
- Marrazzo JM (2016) Epidemiology of vaginal microbiota among women in the United States. J Clin Microbiol 54(6): 1462-1469.
- Hardy L, Jespers V, Van den Bulck M, Buyze J, Mwambarangwe L, et al. (2019) The presence of bacterial vaginosis is associated with more lactobacillus crispatus and odour, but less frequent Candida and Mycoplasma detection. Acta Obstet Gynecol Scand 98(9): 1157-1165.
- Castro J, Machado D, Cerca N (2020) Unveiling the role of Gardnerella vaginalis in polymicrobial bacterial vaginosis biofilms: the impact of other vaginal pathogens living as neighbors. ISME J 14(10): 2590-2605.
- Castro J, Machado D, Cerca N (2019) The role of Gardnerella vaginalis in the pathogenesis of bacterial vaginosis: a conceptual model. J Infect Dis 220(9): 1399-1405.
- Swidsinski A, Mendling W, Loening-Baucke V, Ladhoff A, Swidsinski S, et al. (2005) Adherent biofilms in bacterial vaginosis. Obstet Gynecol 106(5 Pt 1): 1013-1023.
- Anderson DJ, Marrazzo JM (2015) Interaction of Gardnerella vaginalis and HIV infection: implications for morbidity and mortality. Curr HIV/AIDS Rep 12(2): 202-208.
- Castelló J, Toft C, Alonso C, Rodés A, Ballesteros AL, del Cuerpo JF, et al. (2007) Quantitative and qualitative assessment of bacterial vaginosis in pregnant adolescents. Acta Obstet Gynecol Scand 86(12): 1478-1484.
- Bradshaw CS, Tabrizi SN, Fairley CK, et al. (2006) The association of Atopobium vaginae and Gardnerella vaginalis with bacterial vaginosis and recurrence after oral metronidazole therapy. J Infect Dis 194(6): 828-836.
- Tachedjian G, Aldunate M, Bradshaw CS, Cone RA, The role of lactic acid production by probiotic Lactobacillus species in vaginal health.
- Srinivasan S, Liu C, Mitchell CM, Fiedler TL, Thomas KK, et al. (2015) Temporal variability of human vaginal bacteria and relationship with bacterial vaginosis. PloS one 10(7): e0133632.
- Martin DH, Marrazzo JM, Hillier SL (2019) Bacterial vaginosis and the risk of HIV acquisition: a systematic review and meta-analysis. AIDS 33(12): 1865-1872.
- Bradshaw CS, Sobel JD (2016) Current treatment of bacterial vaginosis-limitations and need for innovation. J Infect Dis 214(Suppl 1): S14-S20.
- Workowski KA, Bolan GA (2015) Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep 64(RR-03): 1-137.
- Schwebke JR, Desmond R (2007) A randomized trial of metronidazole in asymptomatic bacterial vaginosis to prevent the acquisition of sexually transmitted infections. Am J Obstet Gynecol 196(6): 517.e1-6.
- Menard JP, Mazouni C, Salem-Cherif I, Florence F, Didier R, et al. (2010) High vaginal concentrations of Atopobium vaginae and Gardnerella vaginalis in women undergoing preterm labor. Obstet Gynecol 115(1): 134-140.
- Hemalatha R, Mastromarino P, Ramalaxmi BA, et al. (2012) Effectiveness of vaginal tablets containing lactobacilli versus pH tablets on vaginal health and inflammatory cytokines: a randomized, double-blind study. Eur J Clin Microbiol Infect Dis 31(11): 3097-3105.
- Anukam K, Osazuwa E, Ahonkhai I, Balakrishna NV, Sesikeran B, et al. (2006) Augmentation of antimicrobial metronidazole therapy of bacterial vaginosis with oral probiotic Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14: randomized, double-blind, placebo-controlled trial. Microbes Infect 8(6): 1450-1454.
- Swidsinski A, Mendling W, Loening-Baucke V, Ladhoff A, Swidsinski S, et al. (2005) Adherent biofilms in bacterial vaginosis. Obstet Gynecol 106(5 Pt 1): 1013-1023.
- Srinivasan S, Fredricks DN (2008) The human vaginal bacterial biota and bacterial vaginosis. Interdiscip Perspect Infect Dis 2008: 750479.
- Alves P, Castro J, Sousa C, et al. (2020) Gardnerella vaginalis outcompetes 29 other bacterial species isolated from patients with bacterial vaginosis, using in vitro Front Cell Infect Microbiol 10(4): 521580.
- Bradshaw CS, Walker SM, Vodstrcil LA, et al. (2019) The influence of the vaginal microbiota on the susceptibility to sexually transmitted infections. J Infect Dis 219(5): 785-792.
- Vaneechoutte M, Guschin A, Simaey VL, et al. (2020) Diagnostic performance of self-collected vaginal specimens for bacterial vaginosis in women attending the gynecological clinic. J Clin Med 9(10): 3223.
- Mitchell C, Balkus JE, Fredricks D (2021) Are we closer to an effective bacterial vaginosis screening test and vaccine? Clin Microbiol Rev 34(1): e0010720.
- Anderson MR, Klink K, Cohrssen A (2004) Evaluation of vaginal complaints. JAMA 291(11): 1368-1379.
- Workowski KA, Bolan GA (2015) Centres for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep 64(RR-03): 1-137.






























