Adjuvant Chemotherapy Delay After Primary Debulking Surgery for Advanced Ovarian Cancer at a Teaching Hospital in Southern Nigeria
Justina Alegbeleye O1* and Olusegun Biyi-Olutunde2
1Department Of Obstetrics and Gynaecology, University of Port Harcourt Teaching Hospital Rivers State, Nigeria
2Department Of Radiation and Clinical Oncology, University Of Port Harcourt Teaching Hospital, Rivers State, Nigeria
Submission: May 29, 2023; Published: June 06, 2023
*Corresponding author: Justina Alegbeleye O, Department of Obstetrics and Gynaecology, University of Port Harcourt Teaching Hospital Rivers State, Nigeria
How to cite this article: Justina Alegbeleye O, Olusegun Biyi-Olutunde. Adjuvant Chemotherapy Delay After Primary Debulking Surgery for Advanced Ovarian Cancer at a Teaching Hospital in Southern Nigeria. J Gynecol Women’s Health 2023: 25(2): 556159. DOI: 10.19080/JGWH.2023.25.556159
Abstract
Background: Treatment of ovarian cancer involves a combination of surgery with optimal debulking surgery followed by chemotherapy, because many women are diagnosed at an advanced stage. The optimal timing of postoperative chemotherapy for ovarian cancer remains poorly defined.
Objectives: To determine the proportion of patient with delayed chemotherapy, the time-to-commencement of adjuvant chemotherapy, and to identify the predictors of delayed chemotherapy among women with advanced ovarian cancer at the University of Port Harcourt Teaching Hospital (UPTH).
Material and Methods: This was a prospective cross-sectional study of 57 patients who underwent surgery for advanced ovarian cancer between January 1, 2018, and December 31, 2022. A proforma was used to obtain the socio-demographic and clinical characteristics of the participants. Chemotherapy delay was defined as initiation of multiagent chemotherapy >42 days from primary debulking surgery. Associations of socio-demographic and clinical characteristics with adjuvant chemotherapy delay were evaluated with multivariate logistic regression.
Results: Most 20 (35.1%) were aged 45-54 years, with a mean age of 46.9 ± 11.3 years. Majority 37 (64.9%) were married and 31 (54.4%) had tertiary education. The most common histological type was serous 36 (52.6%) followed by mucinous 9 (15.8%). Almost all 55 (96.5%) the women had ascites on presentation, all the women were referred to the study centre, with many 33 (57.9%) of the referrals from private clinics. Many of these patients 46 (84%) had their adjuvant chemotherapy delayed, with 39 (69%) presenting with stage III disease. Financial constraint 35 (51.5%) was the main reason for delayed chemotherapy. Menopause was significantly associated with the delay in adjuvant chemotherapy (chi-square = 7.14, p = 0.01). However, multivariable logistic regression analysis did not identify any potential predictors as a factor for delay in commencing adjuvant chemotherapy. For every unit increase in age of these women, there was an insignificant 12% reduced odds of having delayed adjuvant chemotherapy.
Conclusion: Delay in starting adjuvant chemotherapy is known to be a risk factor for overall survival. Being able to identify the causes of these delays will assist healthcare professionals in understanding and mitigating the risk.
Keywords: Ovarian cancer; Adjuvant chemotherapy; Predictors of delay; Surgery; Nigeria
Introduction
Ovarian cancer is the third most common gynaecological cancer and has the worst prognosis. In 2020, about 313,959 new cases and 207,252 deaths were reported globally, with an age-standardized incidence rate of 6.6 per 100,000 person-years [1]. According to research conducted in Nigeria and Africa, ovarian cancer ranks as the second most common gynaecological cancer, comprising approximately 7 to 8.2% of all gynecological malignancies [2]. Unfortunately, around 75% of cases are diagnosed at advanced stages, which can be attributed to the absence of specific clinical manifestations and effective screening methods [3,4]. The survival rate of patients with ovarian cancer is highly dependent on the stage at diagnosis. For stages I and II, relative survival at five years was 89% and 70%, respectively [5,6]. In contrast, for stages III and IV, relative survival at five years was much lower at 36% and 17%, respectively. At ten years, the survival rates were 84%, 59%, 23%, and 8% for stages I, II, III, and IV, respectively [7-9].
Primary debulking surgery (PDS) followed by adjuvant chemotherapy is the standard of care for advanced ovarian cancer. Adjuvant chemotherapy after PDS aims to eliminate residual cancer cells and reduce the risk of disease recurrence [10,11]. However, there is ongoing debate regarding the optimal timing of adjuvant chemotherapy after PDS. Some studies have suggested that delaying adjuvant chemotherapy after PDS may have a negative impact on patient outcomes [12,13]. The rationale behind this is that delaying chemotherapy may allow residual cancer cells to continue growing and potentially lead to disease progression [6,14]. On the other hand, there is also concern that starting chemotherapy too soon after surgery may delay wound healing and increase the risk of postoperative complications. Although many studies have been conducted, no consensus has been reached on the optimal timing for adjuvant chemotherapy after PDS for advanced ovarian cancer.
While the optimal timing of chemotherapy in ovarian cancer is unknown, it has been suggested that delaying chemotherapy beyond 4 weeks may have a negative prognostic impact [15]. However, most large studies have recommended adjuvant chemotherapy only after complete recovery from surgery, which takes an average of 42 days (6 weeks) after curative resection of ovarian cancer [16,17]. Feng et al. [18] concluded that a 6-week interval between surgery and chemotherapy had no effect on prognosis. A longer interval between surgery and the start of adjuvant chemotherapy resulted in a 22% decline in overall survival (OS) of ovarian cancer, with a 4% decrease in relative OS for each week of delay in starting adjuvant chemotherapy [19].
Uson et al. [20] reported in a meta-analysis that the time to adjuvant chemotherapy (between 20 and 40 days) after ovarian cancer surgery with curative intent was not associated with an increased risk of disease recurrence or death. This association, however, was influenced by the optimal debulking rate [20]. Clinicians must balance the potential benefits and risks of postponing chemotherapy with the potential benefits of commencing treatment sooner. Ultimately, the timing of adjuvant chemotherapy should be determined by individual patient factors and a multidisciplinary approach. Previous research focused primarily on locally advanced disease and found a significant reduction in OS if adjuvant chemotherapy was delayed for six weeks or more [4,12]. The current study was carried out to evaluate the proportion of patients with delayed chemotherapy and its predictors.
Materials and Methods
Study Area
This study was conducted at the gynaecological unit of the University of Port Harcourt Teaching Hospital (UPTH). The University of Port Harcourt Teaching Hospital is a 988-bed hospital in Alakahia, in Obio-Akpor Local Government Area of Rivers state. It is a tertiary hospital that serves as a referral centre for all levels of healthcare in Rivers state and other neighbouring states including Bayelsa, Imo and Abia. Every week, the gynaecology clinic is open from Monday to Friday, and each clinic session is led by a team of consultants. Patients are evaluated in the clinic before they are admitted into the gynaecogical ward for surgery.
Methods
This was a prospective cross-sectional study of 57 women with histologically confirmed ovarian cancer managed at the University of Port Harcourt Teaching Hospital between January 1, 2018, and December 31, 2022. The purpose of the study was duly explained to the women and an informed consent was obtained. A structured interviewer-administered questionnaire designed for this purpose was used to obtain socio-demographic, reproductive, and clinical characteristics. Tumour stage, histological type, serum levels of tumour markers, type of surgery, time to commencement of adjuvant chemotherapy, chemotherapy regimen, and reasons for delay were also evaluated. Chemotherapy delay was defined as initiation of multiagent chemotherapy >42 days from primary debulking surgery. The questionnaire for each patient was checked for completeness before it was entered into a spreadsheet and analyzed.
Statistical Analysis
Statistical Package for Social Sciences version 25 was used to analyze the data. Descriptive statistics were summarized using frequency, mean and standard deviation. The association of delayed chemotherapy with socio-demographic, reproductive, and clinical characteristics was assessed using the Chi-square test and multivariate logistic regression analysis, with statistical significance determined at p < 0.05 .
Ethical Consideration
Ethical approval for the study was obtained from the research and ethics committee of the University of Port Harcourt Teaching Hospital. A written informed consent was obtained from the participants prior to inclusion into the study.
Result
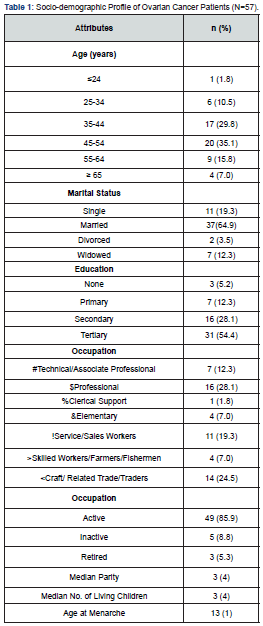
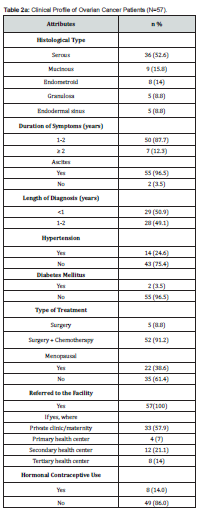
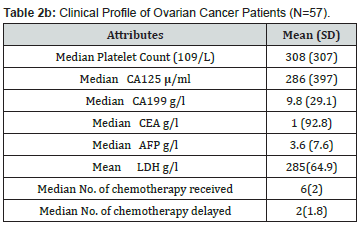
Fifty-seven patients were recruited into the study. Most 20 (35.1%) were aged 45-54 years, with a mean age of 46.9 ± 11.3 years. Majority 37 (64.9%) were married, 31 (54.4%) had tertiary education, and 49 (85.9%) were still active in their respective occupation. This is shown in Table 1. Table 2a shows that the most common histological type was serous 36 (52.6%) followed by mucinous 9 (15.8%) and endometroid 8 (14%). Of the 57 women, 50 (87.7%) had symptoms for 1-2 years, and almost all 55 (96.5%) had ascites at presentation. Most 52 (91.2%) of the women had surgery and chemotherapy. All the women were referred to the study centre, with many 33 (57.9%) of the referrals from private clinics. The median pre-treatment serum CA-125 level was 286 (397) u/ml and a median platelet count of 308 (307) x 109/L, while the median number of adjuvant chemotherapy received was 6(2). This is shown in Table 2b.
SD: Standard deviation
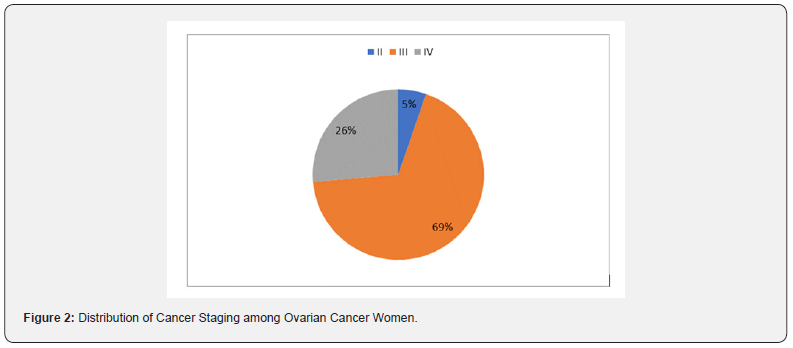
Figure 1 showed that most 46 (84%) of the patients commenced adjuvant chemotherapy more than 42 days after surgery, with financial constraints as the predominant reason for delay (Table 3). Many 39 (69%) of the women had stage III disease, while 15 (26%) of the women had stage IV cancer as shown in Figure 2. Tables 4 & 5 shows univariate analyses used to identify potential predictors of delay in adjuvant chemotherapy. The identified factors were age (median = 48 years, z=2.91 p-value = 0.004), CA 125 (median = 286 μ/ml, z=2.48, p-value = 0.008), platelet count (median = 308 G/L, z=2.46, p-value = 0.014) and menopausal status (X2 = 7.148 p-value = 0.008). However, multivariable logistic regression analysis did not identify any potential predictors as a factor of delay in adjuvant chemotherapy commencement as shown in Table 6. For every unit increase in age, these women had a 12% lower chance of starting adjuvant chemotherapy later. There was no correlation between delayed adjuvant chemotherapy and each unit increase in CA125 in these women. Similarly, there was no association between having delayed adjuvant chemotherapy and every unit increase in platelet count in these women, and menopausal women had an insignificant 105% increased odds of having delayed adjuvant chemotherapy commencement.


Financial + anaemia + medical morbidity + low platelet + poor performance
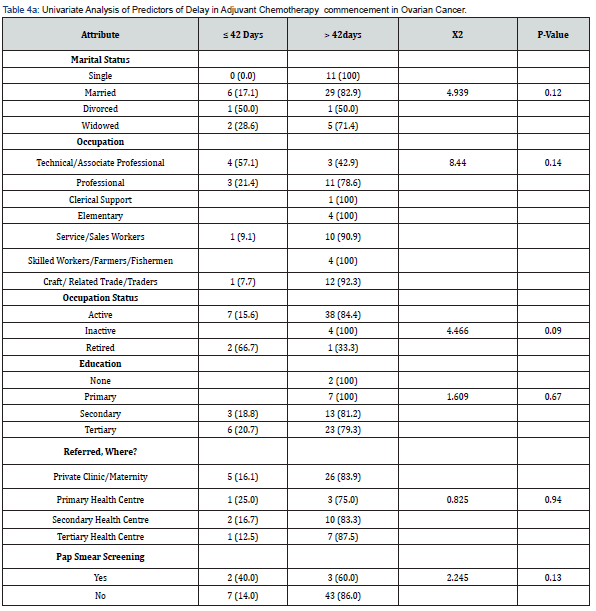
* Significant at p<0.05 in Pearson’s chi-square; ** significant at p<0.05 in Fischer’s exact (>20%Cells <5) X2; Chi-square;

*Significant at p<0.05 in Pearson’s chi-square; ** significant at p<0.05 in Fischer’s exact (>20%Cells <5) X2; Chi-square
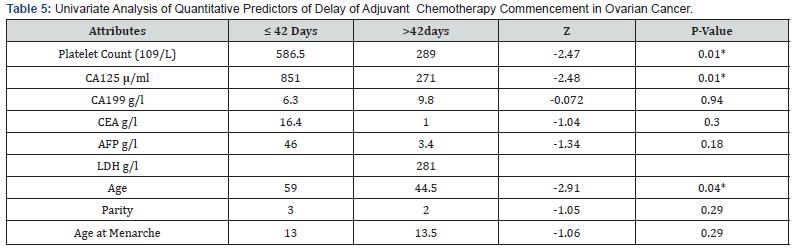
*Significant at p<0.05 Z: Z-score
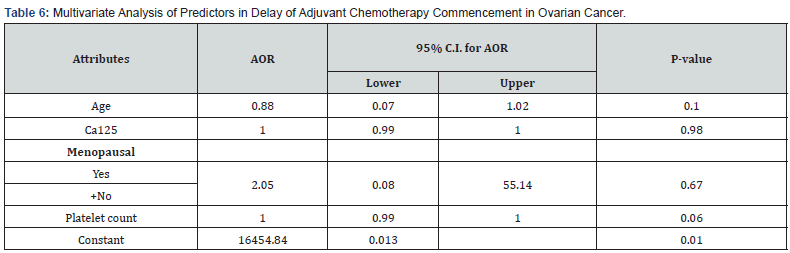
* Significant at p<0.05 AOR: Adjusted Odds Ratio, +: Reference
Discussion
There has been no large-scale research into the timing of adjuvant therapy after optimal debulking surgery. The timing of adjuvant chemotherapy in patients undergoing primary cytoreductive surgery has frequently been debated. Several studies have found that starting chemotherapy after immediate cytoreductive surgery is associated with poor outcomes [21]. However, there is no set time for starting postoperative adjuvant therapy in patients who have had primary cytoreductive surgery. The Gynaecologic Oncology Group (GOG) concluded in their study that patients with a therapy initiation time of more than 25 days had a worse prognosis than those who started therapy earlier [7].
A meta-analysis of 15 cohort studies concluded that starting chemotherapy early improved the overall survival of patients with ovarian cancer [19]. Each week that adjuvant chemotherapy was delayed resulted in a 4% decrease in relative OS. The GOG performed a post-trial ad hoc analysis on ovarian cancer patients from a phase III randomized control trial and found that when the time to chemotherapy exceeded 25 days, there was an increased risk of death in patients with Stage IV disease who had complete resection [7]. The time between surgery and the start of chemotherapy has varied between studies. Most studies investigated a 4-6-week delay. Although some research have been undertaken to determine the optimal time for initiation of adjuvant chemotherapy after interval cytoreductive surgery, no consensus has been reached.
Many of the women in the current study had delayed adjuvant chemotherapy, which was defined as a period of more than 42 days. This is consistent with previous reports from similar studies, which found that 60-90% of women had delayed chemotherapy after surgical removal of ovarian tumours, particularly in low and middle-income countries like Nigeria [7,10,12,22]. This is in contrast to reports from developed countries, which showed that approximately half of women who underwent optimal debulking surgery for advanced ovarian cancer had delayed chemotherapy [14,23]. The high proportion of women who had delayed chemotherapy in the study does not necessarily imply a causal relationship between delayed treatment and lower socioeconomic status.
Chemotherapy delays may be influenced by factors such as age, patient comorbidities, cancer stage, treatment preferences, prolonged hospitalization, postoperative complications, or access to healthcare. Furthermore, the reasons for delayed chemotherapy may differ depending on the healthcare system, the patient’s preferences, and the characteristics of the tumour [24,25]. However, only menopausal status was found to be significantly associated with adjuvant chemotherapy delay in the current study. The finding that many of the women had delayed adjuvant chemotherapy is a cause for concern, as treatment delays can have a negative impact on treatment outcomes and overall survival. Possible reasons for the delay in chemotherapy initiation should be investigated and addressed, including access to healthcare, patient education, and decision-making by physicians.
Many of the women had advanced cancer (stages III and IV). This is consistent with findings from similar studies, in which more than half of women undergoing primary debulking surgery (PDS) presented late [24,26]. This highlights the need for more effective early detection and screening methods to improve the chances of successful treatment outcomes and survival [4,27]. This could include raising awareness and educating women and healthcare providers about the symptoms of ovarian cancer and the importance of early detection. Further research is needed to understand the barriers to timely treatment in this group of women [24,26,28]. Strategies addressing the specific needs of ovarian cancer patients, such as managing treatment-related symptoms, may help to improve treatment adherence and outcomes.
The absence of significant predictors of delayed adjuvant chemotherapy initiation suggests that multiple factors may be contributing to treatment delays. This emphasizes the importance of a multidisciplinary approach to cancer care, including coordinated efforts among healthcare providers, patients, and their families to improve treatment adherence and outcomes. The findings of the current study emphasize the significance of ovarian cancer screening, early diagnosis, and timely treatment.
Addressing the specific needs of women with ovarian cancer, as well as identifying and removing barriers to timely treatment initiation, are critical to improving treatment outcomes for women with ovarian cancer.
The limitations of our study were the small number of patients and the short duration of follow-up. A larger cohort could have achieved statistical significance. Given that this was not a randomized study, there was no prior calculation of sample size or power. The significance of our study was that it was the first time that delayed adjuvant chemotherapy was evaluated. Furthermore, as a single centre, a defined patient selection and optimal debulking surgery protocol was implemented.
Conclusion
The present study observed that majority of the women had delay in the initiation of adjuvant chemotherapy, which was mainly due to financial constraint. Concerted efforts should be made by the government, policy makers, and non-governmental organizations to subsidize cancer care, and make chemotherapy and radiotherapy part of the National Health Insurance Scheme. Furthermore, raising awareness and educating women and healthcare providers about the symptoms of ovarian cancer and the importance of early detection larger studies are needed to identify reversible risk factors that could impact on patient outcomes.
Acknowledgment
The authors hereby acknowledge all the women with advanced ovarian cancer managed at the study centre during the period under review who despite their pains, discomfort, personal struggles, and challenges participated in the study. We salute your courage.
References
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, et al. (2021) Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71(3): 209-249.
- Okunade KS, Adetuyi IE, Adenekan M, Ohazurike E, Anorlu RI, et al. (2020) Risk predictors of early recurrence in women with epithelial ovarian cancer in Lagos, Nigeria. Pan Afr Med J 36: 272.
- Lin H, Chen WH, Wu CH, Ou YC, Chen YJ, et al. (2021) Impact of the Time Interval Between Primary Debulking Surgery and Start of Adjuvant Chemotherapy in Advanced Epithelial Ovarian Cancer. Cancer Manag Res 13: 5413-5422.
- Joseph N, Clark RM, Dizon DS, Lee MS, Goodman A, et al. (2015) Delay in chemotherapy administration impacts survival in elderly patients with epithelial ovarian cancer. Gynecol Oncol 137(3): 401-405.
- Nasioudis D, Kim S, Rubin E (2018) Determining the window for preservation: delay in adjuvant chemotherapy administration is not associated with worse outcomes for young women with stage I epithelial ovarian cancer. Fertil Steril 110: e175.
- Starbuck KD, Szender JB, Duncan WD, Morrell K, Etter JL, et al. (2018) Prognostic impact of adjuvant chemotherapy treatment intensity for ovarian cancer. Plos One 13(11): e0206913.
- Tewari KS, Java JJ, Eskander RN, Monk BJ, Burger RA (2016) Early initiation of chemotherapy following complete resection of advanced ovarian cancer associated with improved survival: NRG Oncology/Gynecologic Oncology Group study. Ann Oncol 27(1): 114-121.
- Ekmann-Gade AW, Schnack TH, Seibæk L, Noer MC, Høgdall C, et al. (2023) Impact of surgery and chemotherapy timing on outcomes in older versus younger epithelial ovarian cancer patients: A nationwide Danish cohort study. J Geriatr Oncol 14(1): 101359.
- Larsen E, Blaakær J (2009) Epithelial ovarian cancer: Does the time interval between primary surgery and postoperative chemotherapy have any prognostic importance? Acta Obstet Gynecol Scand 88(4): 373-377.
- Seagle BL, Butler SK, Strohl AE, Nieves-Neira W, Shahabi S, et al. (2017) Chemotherapy delay after primary debulking surgery for ovarian cancer. Gynecol Oncol 144(2): 260-265.
- Kim YW, Choi EH, Kim BR, Ko WA, Do YM, et al. (2017) The impact of delayed commencement of adjuvant chemotherapy (eight or more weeks) on survival in stage II and III colon cancer: a national population-based cohort study. Oncotarget 8(45): 80061-80072.
- Liu XD, Liu Y, Gong TT, Guo JY, Wang YN, et al. (2018) Prognostic Influence of the Time Interval between Surgery and Chemotherapy in Epithelial Ovarian Cancer. J Cancer 9(22): 4172-4178.
- Somashekhar SP, Ramya Y, Ashwin KR, Shabber SZ, Ahuja VK, et al. (2020) Rohit Evaluation of delay in time to adjuvant chemotherapy after HIPEC and its impact on oncological outcome in advanced epithelial ovarian cancer. Pleura and Peritoneum 5(3): 20200103.
- Seebacher V, Reinthaller A, Koelbl H, Concin N, Nehoda R, et al. (2017) The Impact of the Duration of Adjuvant Chemotherapy on Survival in Patients with Epithelial Ovarian Cancer – A Retrospective Study. Plos One 12(1): e0169272.
- Alexander M, Blum R, Burbury K, Coutsouvelis J, Dooley M, et al. (2017) Timely initiation of chemotherapy: a systematic literature review of six priority cancers - results and recommendations for clinical practice. Intern Med J 47(1): 16-34.
- Ozols RF, Bundy BN, Greer BE, Fowler JM, Clarke-Pearson D, et al. (2003) Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol 21(17): 3194-3200.
- Katsumata N, Yasuda M, Takahashi F, Isonishi S, Jobo T, et al. (2009) Dose-dense paclitaxel once a week in combination with carboplatin every 3 weeks for advanced ovarian cancer: a phase 3, open-label, randomized controlled trial. Lancet 374(9698): 1331-1338.
- Feng Z, Wen H, Bi R, Yang W, Wu X, et al. (2016) Prognostic impact of the time interval from primary surgery to intravenous chemotherapy in high grade serous ovarian cancer. Gynecol Oncol 141(3): 466-470.
- Liu Y, Zhang T, Wu Q, Jiao Y, Gong T, et al. (2017) Relationship between initiation time of adjuvant chemotherapy and survival in ovarian cancer patients: a dose-response meta-analysis of cohort studies. Sci Rep 7(1): 9461.
- Usón PLJ, Bugano DD, França MS, Antunes YP, Taranto P, et al. (2017) Does Time-to-Chemotherapy Impact the Outcomes of Resected Ovarian Cancer? Meta-analysis of Randomized and Observational Data. Int J Gynecol Cancer 27(2): 274-280.
- Lee YY, Lee JW, Lu L, Xu W, Kollara A, et al. (2018) Impact of interval from primary cytoreductive surgery to initiation of adjuvant chemotherapy in advanced epithelial ovarian cancer. Int J Gynaecol Obstet 143(3): 325-332.
- Adejumo R (2020) 339 Ovarian cancer epidemiology in Jigawa, Nigeria. A 4-year review. Int J Gynaecol Cancer 30.
- Rocher G, Gaillard T, Uzan C, Collinet P, Bolze PA, et al. (2021) Does Time-to-Chemotherapy after Primary Complete Macroscopic Cytoreductive Surgery Influence Prognosis for Patients with Epithelial Ovarian Cancer? A Study of the FRANCOGYN Group. J Clin Med 10(5): 1058.
- Zayyan MS, Ahmed SA, Oguntayo AO, Kolawole AO, Olasinde TA, et al. (2017) Epidemiology of ovarian cancers in Zaria, Northern Nigeria: a 10-year study. Int J Women's Health 9: 855-860.
- Jones H, Coyne P, Evans M (2015) PWE-330 Causes for delay in commencing adjuvant chemotherapy in rectal cancer. Gut 64: A355.2-A355.
- George S, Omotoso A, Pinto A, Mustapha A, Sanchez-Covarrubias AP, et al. (2021) An Assessment of Ovarian Cancer Histotypes Across the African Diaspora. Front Oncol, p. 11.
- Joneborg U, Palsdottir K, Farm E, Johansson H, Salehi S, et al. (2021) Time-interval to adjuvant chemotherapy and postoperative management after upper abdominal surgical procedures in advanced ovarian cancer. Eur J Surgl Oncol 47(2): 353-359.
- Onyiaorah IV, Anunobi CC, Banjo AA, Fatima AA, Nwankwo KC, et al. (2011) Histopathological patterns of ovarian tumours seen in Lagos University Teaching Hospital: a ten-year retrospective study. Nig Q J Hosp Med 21(2): 114-118.






























