Distribution of human papillomavirus genotypes and potential impact of nonavalent HPV vaccination in the Lorraine Region
Bérénice Fanjeaux1, Thierry Routiot1, Béatrice Marie2, Philippe Judlin1,4 and Véronique Venard3,4*
1Department of Gynecological and Obstetrical, CHRU Nancy, Vandoeuvre-lès-Nancy-Cedex, France
2Department of Biopathology, CHRU Nancy, Vandoeuvre-lès-Nancy-Cedex, France
3Laboratory of Virology, CHRU de Nancy, Vandoeuvre-lès-Nancy-Cedex, France
4Faculty of Medicine, Lorraine University, Vandoeuvre-lès-Nancy-Cedex, France
Submission: December 15, 2021; Published: January 03, 2022
*Corresponding author: Véronique Venard, Faculty of Medicine, Lorraine University, Vandoeuvre-lès-Nancy-Cedex, France
How to cite this article: Bérénice F, Thierry R, Béatrice M, Philippe J, Véronique V. Distribution of human papillomavirus genotypes and potential impact of nonavalent HPV vaccination in the Lorraine Region. J Gynecol Women’s Health 2022: 22(5): 556099. DOI: 10.19080/JGWH.2022.22.556099
Abstract
Background: Epidemiological data on Human Papillomavirus (HPV) genotypes distribution according to territories are essential to estimate the potential impact of HPV vaccination. To our knowledge, these are the first data available in Lorraine concerning the local epidemiology of HPV genotypes found according to cytological and histological abnormalities. These data were compared with existing French data on the distribution of HPV genotypes in genital lesions (EDITH studies).
Material and Methods: Cervical samples of women were tested for HPV DNA detection and genotyping. These included 322 samples with atypical squamous cells of undetermined significance (ASCUS), 82 atypical squamous cells suspected of high grade lesions (ASC-H), 100 low-grade squamous intraepithelial lesions (LSIL), and 19 high-grade squamous intraepithelial lesions (HSIL).
Results: The most common genotype found was HPV 16 with the following distribution: 17.1% in ASCUS, 32.9% in ASC-H, and 31.6% in HSILs, followed by 13.4% and 26.3% HPV 31 in ASC-H and in HSIL, respectively. Thus, the prevalence of High-Risk HPV (HR HPV) 16 increases with the potential severity of cytological lesions and precancerous histological lesions (22 %, 41% and 56% in Cervical intraepithelial neoplasia, respectively grade 1, grade 2, grade 3). The prevalence of additional HR HPV 16/18 and HPV 31/33/45/52/58 follows the same trend, with an increase parallel to the potential severity of smear lesions (48% in ASCUS; 63% in HSIL) and anatomopathological abnormalities (63% in CIN1; 66% in CIN2 and 77% in CIN3).
Conclusion: Our study confirmed the high local prevalence of HPV 16, an under-representation of types 18 and 58 in favor of genotypes HPV 31, HPV 51 and HPV 52 Nonavalent vaccination could prevent 42.0 to 84.2% of pathological smears (ASCUS, ASC-H, LSIL and HSIL) and prevent 62,7 to 83.6% of low-grade lesions (CIN1) and 70.3 to 84.7% of high-grade lesions (CIN2 / 3).
Abbreviations: HPV: Human Papillomavirus; ASCUS: Atypical Squamous Cells Of Undetermined Significance; LSIL: Low-Grade Squamous Intraepithelial Lesions; HSIL: High-Grade Squamous Intraepithelial Lesions; CIN: Cervical Intraepithelial Neoplasia; STI: Sexual Transmitted Infection
Introduction
Cervical cancer due to high risk human papillomavirus (HR HPV) is the second leading cause of cancer-related death in women worldwide (288,000 deaths per year). In France, there are approximately 3,000 new cases and nearly 1,000 deaths per year due to this cancer [1].
Two prophylactic vaccines have been available in France since 2007. A nonavalent vaccine (against HPV 6, 11, 16, 18 and 31, 33, 45, 52, 58) received an European authorization in 2015, and the Haut Conseil de Santé Publique recommended its use in France in 2017 [2]. Prevalence of HPV genotypes may vary from one country to another and it is important to benefit from data both up-to-date and accurate on the distribution of genotypes in the most common genital diseases associated with HPV, including in the perspective of HPV vaccination setting up [3]. France is the only country that have been set up a large HPV genotyping program with the EDiTH studies (for “Study the distribution of HPV types “) since 2006 [4-7].
The EDITH studies were published in France analyzing the distribution of HPV including different centers according to the study period. For EDITH I, eleven centers, and for EDITH II and III, 3 centers participated. The Lorraine Region did not participate in these studies. Every HPV distribution studies in France involved around 400 to 500 samples, with an overall HPV prevalence close to 98%. The main objective of this study was to investigate the local epidemiology of HPV genotypes in the Lorraine Region, their distribution according to cytological and histological abnormalities and to compare it with the national data of the EDITH studies. The secondary objective was to evaluate the potential impact of a nonavalent anti-HPV vaccination in the Lorraine Region.
Materials and Methods
Studied Population
The study was retrospective, observational, single-center (Nancy University Hospital).All patients (n=635) who underwent HPV genotyping were included at the Virology laboratory of the CHRU in Nancy, from 2014 to 2017 on a sample of genital sphere. The patients who received an anonymous HPV genotyping because they were managed by the Planning Familial (N=15), or an HPV genotyping on a specimen other than a genital specimen (N=22) were not included. The patients whose age did not correspond to the recommendations for cervical cancer screening (<25 years and> 65 years) (N=49) and the patients not residing in Lorraine (N = 20) were not included. So, 529 patients meeting the eligibility criteria were included.
Data Collection
Data were collected on socio-demographic and medical characteristics, including gyneco-obstetrical characteristics (pregnancy, parity, contraception, HPV vaccination), as well as cytological, virological and anatomopathological data. Comorbidities were looked for, such as the smoking, alcohol or drug uses, the presence of a sexual transmitted infection (STI) (HIV, Chlamydiae, Gonococcus) or the taking of immunosuppressive therapy.
Smears were taken in the BD SurePath® liquid medium, allowing immunohistochemistry and molecular testing to be performed. All genotyping was performed at the Virology laboratory of the CHRU of Nancy, using the Fujirebio INNOLipa HPV Genotyping Extra II kit, a PCR SFP10 PCR genotyping technique with hybridization on strips. The kit specifically looks for:
a) 13 High Risk Human Papillomavirus (HR HPV): 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68.
b) 6 HPV potentially High Risk (HR): 26, 53, 66, 70, 73, 82
c) 13 Low Risk Human Papillomavirus (LR HPV): 6, 11, 40, 42, 43, 44, 54, 61, 62, 67, 81, 83, 89
Statistical Analyses
A descriptive analysis was performed on the entire study population. The results were presented in the form of numbers and percentages for qualitative variables, and means and standard deviations for quantitative variables.
Ethical Considerations
After validation of the study by the Research and Innovation Department of the Nancy CHRU, a simple declaration to the Commission Nationale de l’Informatique et des Libertés (France) was made by the Institution’s Data Protection Officer.
Result
We studied the data collected in a descriptive way, with the aim of characterizing the studied population and determining its HPV epidemiology. Then we compared these results with those obtained at the national level (EDITH studies) [4-7].
Description of the Studied Population
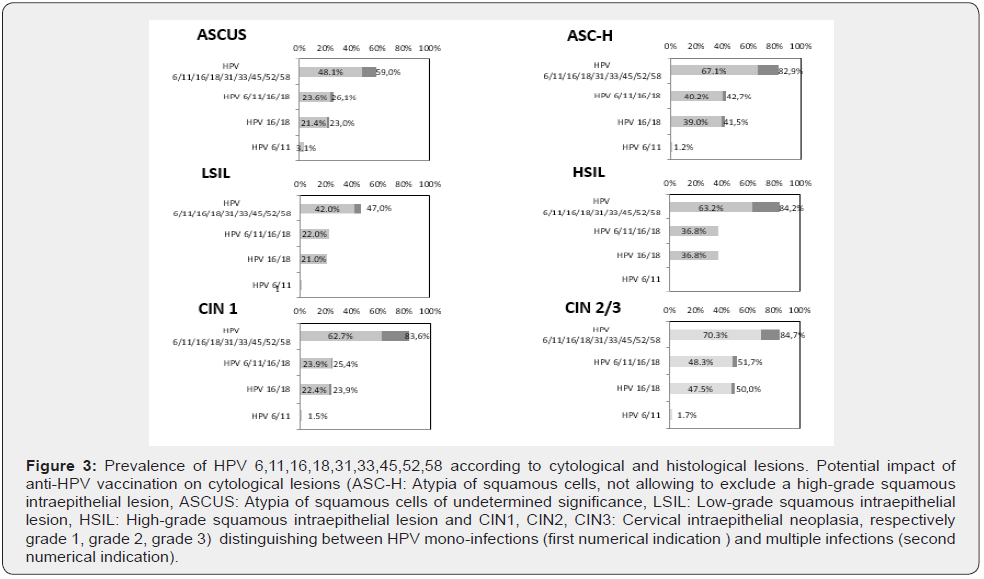
The characteristic of the population is presented in Table1.
Among the 635 patients who underwent HPV genotyping over the study period (2014-2017), 529 were included. The age of our population was on average 39.3 +/- 10.9 years. Concerning the gynecological obstetrical data, the gestity was 2 for an average parity of 1.5 with approximately one abortion for 1 patient out of 5. The gynecological follow-up of these women were described as regular (1 consultation / year) for 519 of them (98%). Only 13/529 women were vaccinated against HPV (full Gardasil® regimen), i.e.,less than 2.5% of our population. The contraceptive method has also been sought (Table I).
Study of the Distribution of HPV Genotypes in Cytological Lesions
The overall prevalence of different HPV types was calculated for each type of cytological lesion (Figure 1). Only the 15 most important HPV genotypes were reported.
Among the 529 inclusions, 322 cases of Atypical Squamous Cells of Undetermined Significance ASCUS (60.9%) could be analyzed. The median age at diagnosis was 37 years old. At least one HPV strain was detected in 256/322 cases (79.5%) and the infection was multiple in 103/322 of cases (32%). HPV 16 was the most frequent genotype (56/322, 17.6%), followed by HPV 52 (39/322, 12.1%), HPV 53 (27/322, 8.4%), HPV 51 (27/322, 8.4%) and HPV 31 (29/322, 8.1%).
There were 82 cases of Atypical Squamous Cells suspecting High grade lesions (ASC-H) (15.5%). The median age at diagnosis was 39.5 years. At least one HPV strain was detected in 77/82 cases (93.9%) and the infection was multiple in 32/82 cases (39%). HPV 16 was the most frequent genotype (27/82, 32.9%), followed by HPV 31 (11/82, 13.4%), HPV 51 (10/82, 12.2%), HPV 52 (8/82, 9.7%) and HPV 66 (7/82, 8.5%).
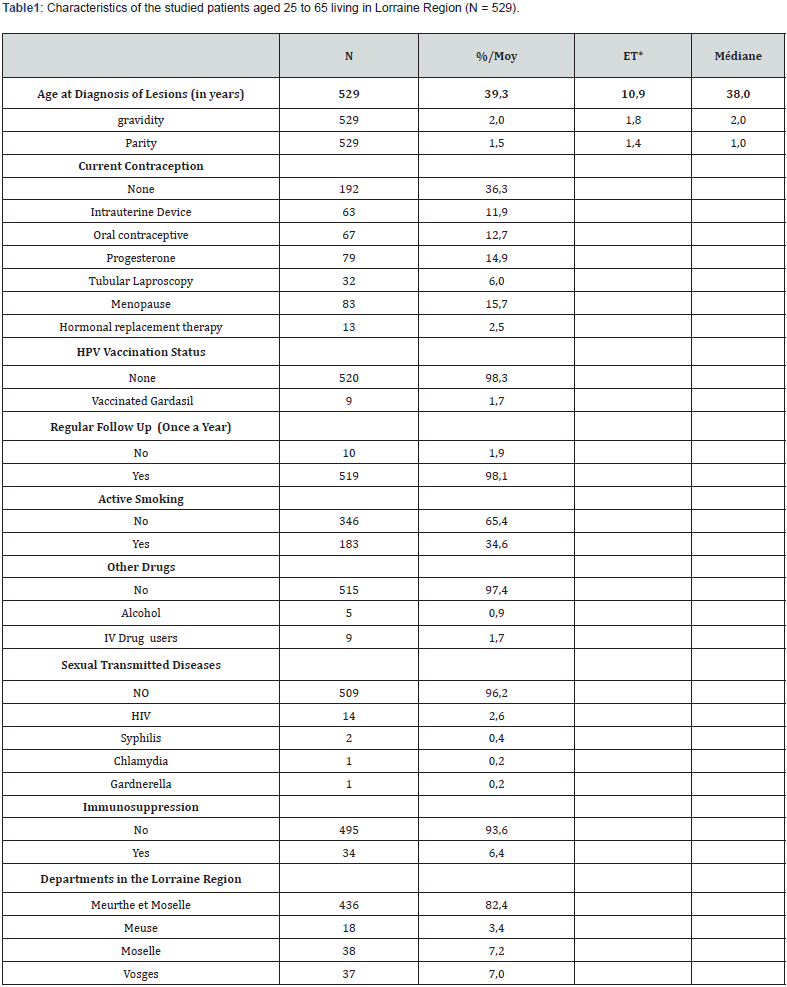
100 cases of Low-Grade Squamous Intraepithelial Lesions (LSIL) (18.9%) were analyzed. The median age at diagnosis was 42 years old. The presence of HPV was found in 82/100 cases (82%) and multiple infection in 31% of cases (31/100). HPV 53 was the most frequent (18/100, 18.0%), followed by HPV 16 (16/100, 16.0%), HPV 66 (14/100, 14.0%), HPV 52 (7/100, 7.0%) and HPV 31 (7/100, 7.0%).
There were 19 cases of High-Grade Squamous Intraepithelial Lesions (HSIL) (3.6%). The median age at diagnosis was 32 years old. The HPV was found in 18/19 cases (95%) and multiple infection in half of the cases (10/19). HPV 16 was the most frequent (6/19, 31.6%), followed by HPV 31 (5/19, 26.3%), HPV 68 (2/19, 10.5%), HPV 51 (2/19, 10.5%) and HPV 39 (2/19, 10.5%). We could note active smoking for 183/529 of them (35%), drug addiction for 9/529 (1.7%) and alcohol addiction for 5/529 (1%).
Study Of The Distribution Of HPV Genotypes In Histological Lesions
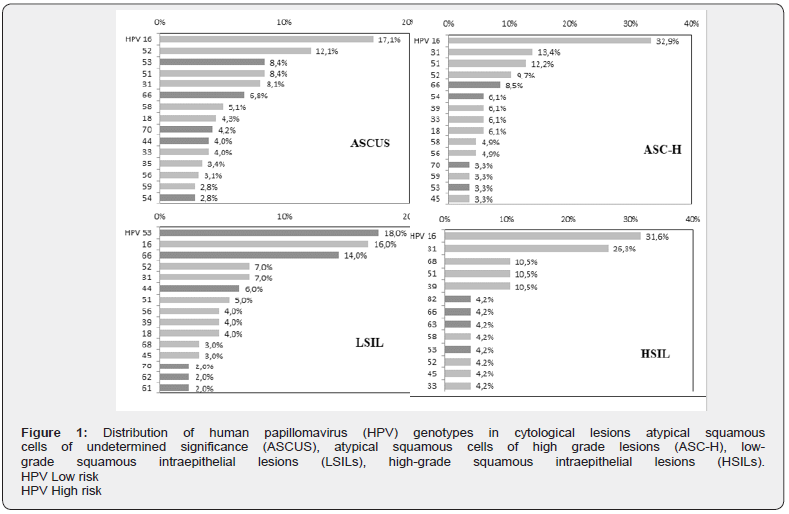
The overall prevalence of different types of HPV was calculated for each histological lesion. Only the 15 most important HPV genotypes were reported (Figure 2).
67 cases of Cervical Intraepithelial Neoplasia of grade 1 (CIN 1) (23.2%) were analyzed. The median age at diagnosis was 36.8 years. At least one HPV strain was detected in 64/67 cases (95.5%) and the infection was multiple in 32/67 cases (47.7%). HPV 31 was the most frequently found (15/67, 22.4%), followed by HPV 52 (13/67, 19.4%), HPV 16 (10/67, 14.9%) and HPV 51 (9/67, 13.4%).
There were 70 cases of Cervical Intraepithelial Neoplasia of grade 2 (CIN 2) (24.2%). The median age at diagnosis was 33.7 years. The presence of HPV was found in 60/70 cases (85.7%) and multiple infection in 34.3% of cases (24/70). HPV 16 was the most frequent (23/70, 32.8%), followed by HPV 51 (8/70, 11.4%), HPV 52 (6/70, 8.6%), HPV 58 (4/70, 5.7%) and HPV 31 (4/70, 5.7%).
48 cases of Cervical Intraepithelial Neoplasia of grade 3 (CIN 3) (16.2%) were analyzed. The median age at diagnosis was 37.4 years. At least one HPV strain was detected in 46/48 cases (89.6%) and the infection was multiple in 41.6% of cases (20/48). HPV 16 was the most frequent (18/48, 37.8%), followed by HPV 31 (5/48, 10.4%), HPV 33 (3/48, 6.3%), HPV 52 (3/48, 6.3%) and HPV 51 (3/48, 6.3%).
Distribution of Genotypes in HPV Vaccinated Patients
The HPV genotypes found in the 13/529 patients, who received a complete regimen of 3 injections of Gardasil®, were verified. HPV 52 (7/13), HPV 51 (4/13), HPV 31 (4/13), HPV 66 (2/13) and HPV 68 (1/13) were detected. Histology was available for 9 of these patients. It was normal in 3/9; CIN1 in 3/9 and CIN2 in 3/9. For the 3 CIN2 lesions, HPV 52 was positive. HPV 16 was detected in one patient. Upon investigation, it was found out that the vaccination had been performed out after the first sexual intercourse. The smear was HSIL with HPV 16 and HPV 31 positivity, which is why the patient underwent colposcopy with biopsy afterwards which revealed CIN 1 on histology.
Distribution of HPV Genotypes in Smoking Patients
With a statistically equivalent distribution of cytological and histological lesions, the distribution of the most frequently encountered HPV genotypes appears similar whether the population is marked by active smoking (N = 183) or not (N = 346). The overall prevalence of genotype 16 appears higher in smoking patients: 35% (64/183) vs 24.6% (85/346), followed by HPV 52: 18.0% (33/183) vs 13% (45/346), HPV 53: 13.7% (25/183) vs 11.8% (41/346), and HPV 51: 12.6% (23/183) vs 10.4% (36/346). The overall HPV prevalence is 84.7% (155/183) vs 81.8% (283/346), with multiple infection in 44.1% (15/34) vs 28.5% (141/495) cases.
Distribution of HPV Genotypes in Patients Undergoing Immunosuppressive Treatment
With a statistically equivalent distribution, in terms of cytological and histological lesions, the distribution of the HPV genotypes most frequently encountered in the population treated with an immunosuppressant (N=34) appears different from this observed in the general population (N=495). The most frequently encountered genotype is HPV 53: 26.5% (9/34) vs 11.7% (58/495), then HPV 44: 17.6% (6/34) vs 4.8% (24/495), and HPV 39: 14.7% (5/34) vs 4.4% (22/495) followed by equal prevalence HPV 66, HPV 52, HPV 35, HPV 33 and HPV 18 “f”: 8.9% (3/34). The overall HPV prevalence is 88.2% (30/34) vs 82.4% (408/495), with multiple infection in 47.1% (16/34) vs 30.5% (151/495) cases.
Comparative Data - Comparison of the Prevalence of HPV Genotypes in Our Low-Grade Lesions (LSIL) and Those in The EDITH III Study
The HPV strains most frequently found in LSIL smears (N = 100) in our cohort were HPV 53 (18.0%), followed by HPV 16 (16.0%), 66 (14.0%), 52 (7.0%) and 31 (7.0%). The presence of HPV was found in 82% of these cases (82/100) and multiple infection in 31% of cases (31/100). In the national EDITH III cohort, involving 397 LSIL smears suggestive of low-grade lesions, HPV 66 was the most common (25%) (99/397), followed by HPV 16 (21%) (85 / 397), 53 (18%) (72/397) and 51 (17%) (69/397). A HPV strain was detected in 98.2% (390/397) of the samples with multiple infection in half of the cases (198/397).
Comparison of the Prevalence of HPV Genotypes in Our High-Grade Lesions (CIN2 / 3) and Those in the EDITH II Study
The HPV strains most frequently found in CIN2 / 3 lesions (N = 137) in our cohort were HPV 16 (47/137, 34.3%), followed by HPV 51 (12/137, 8.8%), HPV 31 (11/137, 8.0%), and HPV 52 (9/137, 6.6%). The presence of HPV was found in 119/137 cases (86.9%) and multiple infection in 37.9% of cases (52/137).
In the national EDITH II cohort, involving 493 cases of CIN2 / 3, the presence of HPV was detected in 484/493 cases (98.2%), with multiple infection in 31% of cases (153/493). The most frequent HPV was type 16 (306/493, 62%), followed by HPV 31 (59/493, 15%), HPV 33 (59/493, 12%) and HPV 52 (44/493, 9%).
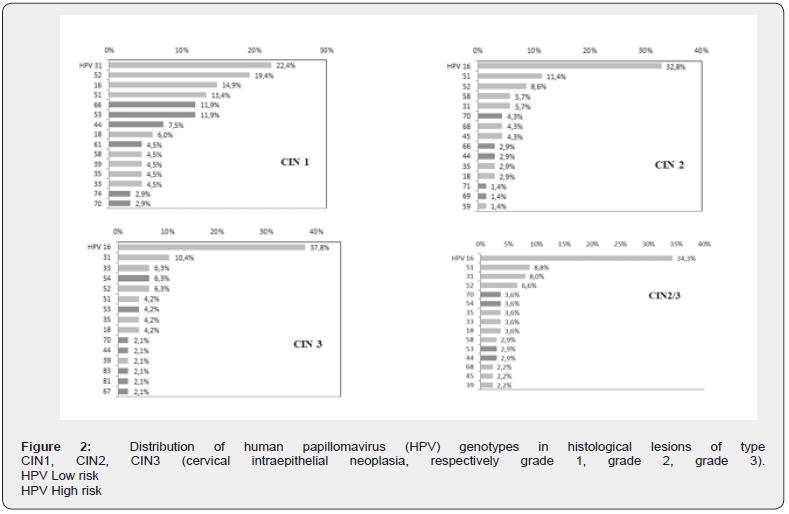
Prevalence of Genotypes 6, 11, 16, 18, 31, 33, 45, 52, and 58
The prevalence of HPV 6/11, HPV 16/18, HPV 6/11/16/18 and HPV 6/11/16/18/31/33/45/52/58 were calculated in each type of cytological lesion (Figure 2) and histological (Figure 3), distinguishing between HPV mono-infections and multiple infections, in order to be able to estimate the minimum and maximum potential impact of the quadrivalent vaccination but also of the nonavalent vaccination available today. Thus, the prevalence of HPV HR 16/18 increases with the potential severity of cytological lesions (21% in ASCUS and LSIL; 39% in ASC-H and nearly 37% in HSIL) and precancerous histological lesions (22 % in CIN1; 41% in CIN2 and 56% for CIN3).The prevalence of additional HR HPV 16/18 + 31/33/45/52/58 follows the same trend, with an increase parallel to the potential severity of smear lesions (48% in ASCUS; 63% in HSIL) and anatomopathological abnormalities (63% in CIN1; 66% in CIN2 and 77% in CIN3)
Discussion
Cervical cancer is the second leading cause of cancer-related death in women worldwide. In France, there are approximately 3,000 new cases and nearly 1,000 deaths per year [1]. However, this virus-induced cancer is avoidable, as it can be prevented by primary prevention (vaccination) and secondary prevention (screening by cervical smear and HPV test) and early management of the precancerous lesions detected. It is estimated that the genotypes 16 and 18 are responsible for 70% of cervical cancers and 50% of high-grade lesions, and that the HPV 31, HPV 33, HPV 45, HPV 52, HPV 58 genotypes (included in the nonavalent vaccine) are responsible for 15 and 20% of cervical cancers and 30 to 40% of high-grade lesions [8]. Nonavalent vaccination could reduce the risk of cervical cancer by 90% of the risk and highgrade lesions by 80% [2,9,10].
The epidemiological data concerning the distribution of HPV genotypes according to territories are essential for estimating the potential impact of HPV vaccination. To our knowledge, these are the first data available in Lorraine concerning the local epidemiology of HPV genotypes found according to cytological (ASCUS, ASC-H, LSIL and HSIL) and histological (CIN1, 2 and 3) abnormalities. These data were compared with the existing French data on the distribution of HPV genotypes in genital lesions (EDITH studies) [4-7, 11].
Our study confirmed the high local prevalence of HPV 16, and the under-representation of types HPV 18 and HPV 58 in favor of genotypes HPV 31, HPV 51 and HPV 52 and the reporting of the frequent presence of HPV 53, HPV 66 and HPV 70 regardless of the severity of the lesions whose uncertain oncogenicity is to be monitored [12].
Among the results of this study, the main finding was the predominant presence of HPV 16 in all high-grade lesions, with an increasing prevalence with the severity of cytological lesions, ranging from 16-17% in ASCUS and LSIL lesions, to 31-33% in ASC-H and HSIL lesions, and to 15% in CIN1, 33% in CIN2 and 38% in CIN3 [12].
The other important finding was the low overall prevalence of HPV 18 (5%) in our population regardless of the lesion, leaving room for certain s HR HPV genotypes, with unexpected prevalence of HPV 31 (26% in HSIL and 10% in CIN3), HPV 51 and HPV 52 found in more than 20% of ASC-H lesions, and 15% of HSIL lesions and CIN2 / 3 lesions. These 3 HR HPV were also found in the cytologies and histologies of patients who received a complete regimen of 3 injections of Gardasil®. The study of the local distribution of HPV also highlighted the presence of HPV 53, 66 and 70 among the 10 most frequent HPV genotypes, regardless of the severity of the cytological or histological lesions. At present, these 3 types of HPV are of uncertain oncogenicity, and classified as probable high risk oncogenes, due to their structural similarity to other oncogenic HPV [13].
The overall prevalence of HPV observed ranged from 80 to 95% depending on the lesions, indicating the causal link, now certain, between HPV and cervical precancerous lesions, but also the sensitivity of HPV DNA detection method used (SPF10 primers and PCR amplification) [14,15]. Our results also confirmed a decrease in the proportion of multiple infections according to the severity of the lesions, with 48% of CIN1 and 38% of CIN2 / 3, a phenomenon reflecting the viral clearance of LR HPV, and the aggressive persistence of HR HPV HR [12,16].
In women with an immunosuppressed background, induced by active smoking, or secondary to immunosuppressive treatment, higher overall HPV prevalence (85-88%) were observed higher than in the general population (81-82%), with more frequent multiple infections (44-47% vs 28-30%) reinforcing the idea of a less effective viral clearance in case of deficient cellular immunity and smoking as a major risk factor in the onset and persistence of HPV infections [3,17].
This observation reminds us the importance of the advice on smoking cessation during consultation and the benefit of vaccinating these populations early on before the initiation of immunosuppressive treatment, as recommended by the Haut Conseil de la Santé Publique [10,13,18-20]. The distribution of the most frequent HPV genotypes in women who smoke was similar to that observed in the general population. It appeared to be different in patients on immunosuppressive therapies [13,18] or HIV seropositivity [19,20], with a significant prevalence of probable high risk HPV 53 (27%), HPV 66 (10%), and HPV 70 (7%), in addition to the HR HPV.
Three cases of HPV 53 mono-infected invasive cervical cancer were reported in the EDITH I cohort, leading to reconsideration of the potential oncogenicity of this genotype [4,18,19]. In HIV seropositive patients, we found the predominant prevalence of HPV 16 (18%) and HPV18 (27%) over other HR HPVs described in the literature [19,20].
The correlation of cytological and histological data was evaluated in order to verify the cyto-histological concordance which is essential for an efficient screening and a good interpretation of our results. Indeed, false-negative cytology results lead to a delay in the management and treatment of existing lesions. False negatives are most due to sampling errors by samplers or to errors in the reading or interpretation of atypical cells in the laboratory. Cytology screening must be sensitive, but also specific. False-positive cytology can lead to overtreatment, with inherent obstetric and psychological morbidity. Histology is not a perfect gold standard either, with diagnostic performance increasing with the severity of the lesions. Almost 28% of ASCUS smears corresponded to a high-grade histological lesion (CIN 2/3). The detection of HPV genotypes has its place here now in screening, allowing stratification of cytological lesions according to the presence or absence of HPV and their potential oncogenicity [12,21,22].
Finally, the potential impact of HPV vaccination was estimated from the prevalence of HPV genotypes 6, 11, 16, 18, 31, 33, 45, 52, and 58, found in each type of cytological and histological lesions. The distinction between single infections and multiple infections was made in order to assess the expected minimum and maximum impact of vaccination. Single infection (or monoinfection) is defined as infection with a single HR HPV genotype. Multiple infection (or co-infection) refers to the presence of more than one HR HPV. Considering 100% vaccination coverage, preexposure vaccination, i.e. no ongoing infection with the relevant HPV genotypes (6, 11, 16, 18 +/- 31, 33, 45, 52, 58), and that smear screening coverage remains constant, quadrivalent vaccination (HPV 6, 11, 16, 18) could prevent 22 to 43% of pathological smears (ASCUS, ASC-H, LSIL and HSIL) and prevent 23, 9 to 25.4% of low-grade lesions (CIN1), and 48.3 to 51.7% of high-grade lesions (CIN2 / 3).
Nonavalent vaccination (HPV 6, 11, 16, 18 and 31, 33, 45, 52, 58) could prevent 42.0 to 84.2% of pathological smears (ASCUS, ASC-H, LSIL and HSIL) and prevent 62,7 to 83.6% of low-grade lesions (CIN1) and 70.3 to 84.7% of high-grade lesions (CIN2 / 3). These estimates are lower than those reported at the national level (25.9 to 56.2% of low-grade lesions and 76.7 to 89.5% of high-grade lesions) with the lower overall prevalence of HPV 16 genotypes, and especially HPV 18, in our local epidemiology [23]. Nonavalent vaccination appears very promising in our population due to a local epidemiology marked by high prevalence of additional genotypes included in the Gardasil® vaccine, with twice more pathological smears avoided and the prevention of 2/3 of histological lesions whatever the grade.
In France, the vaccination coverage rate is among the lowest in Europe (19%) and that screening is carried out by only 60% of the target population in France [24]. Efforts must focus on the acceptance of the vaccine and access to care in order to optimize the effects of these preventive measures which have been underused until now.
Acknowledgment
Thomas Remen
Past adress : Plateforme d’aide à la recherche clinique, Unité de Méthodologie, Data management & Statistiques, CHRU Nancy, Vandoeuvre-lès-Nancy-Cedex, France
Present adress : FACILIT-R, NOM COMMERCIAL : SCIENTIGO, 68 Boulevard Lobau 54000 NANCY
Port. : +33 7 49 70 06 46 contact@scientigo.fr.
References
- https://www.e-cancer.fr/
- Résumé des caractéristiques du produit Gardasil 9®. (s.d.).
- Winer RL, Kiviat NB, Hughes JP, Adam DE, Lee S-K, et al. (2005) Development and duration of human papillomavirus lesions, after initial infection. The Journal of Infectious Diseases 191(5): 731‑
- Prétet J-L, Jacquard A-C, Carcopino X, Monnier-Benoit S, Averous G, et al. (2008) Human papillomavirus genotype distribution in high grade cervical lesions (CIN 2/3) in France : EDITH study. International Journal of Cancer 122(2): 424‑
- Prétet J-L, Jacquard A-C, Saunier M, Clavel C, Dachez R, et al. (2008) Human papillomavirus genotype distribution in low-grade squamous intraepithelial lesions in France and comparison with CIN2/3 and invasive cervical cancer : The EDiTH III study. Gynecologic Oncology 110(2): 179‑
- Aubin F, Prétet J-L, Jacquard A-C, Saunier M, Carcopino X, et al. (2008) Human papillomavirus genotype distribution in external acuminata condylomata : A Large French National Study (EDiTH IV). Clinical Infectious Diseases 47(5): 610‑
- Jacquard A-C, Denis F, Prétet J-L, Aubin F, Pradat P, et al. (2010) Épidémiologie des génotypes de papillomavirus (HPV) dans les lésions anogénitales en France. Médecine thérapeutique / Pédiatrie 13(1): 80‑
- De Vuyst H, Clifford GM, Nascimento MC, Madeleine MM, Franceschi S (2009) Prevalence and type distribution of human papillomavirus in carcinoma and intraepithelial neoplasia of the vulva, vagina and anus : A meta-analysis. International Journal of Cancer 124(7): 1626‑
- St Laurent J, Luckett R, Feldman S (2018) HPV vaccination and the effects on rates of HPV-related cancers. Current Problems in Cancer 42(5): 493‑
- Haut Conseil de la santé publique (2017) Avis relatif à la place du vaccin GARDASIL 9® dans la stratégie actuelle de prévention des infections à papillomavirus humains.
- Abramowitz L, Jacquard A-C, Jaroud F, Haesebaert J, Siproudhis L, et al. (2011) Human papillomavirus genotype distribution in anal cancer in France: The EDiTH V study. International Journal of Cancer 129(2): 433‑
- Guan P, Howell-Jones R, Li N, Bruni L, de Sanjosé S, et al. (2012) Human papillomavirus types in 115,789 HPV-positive women : A meta-analysis from cervical infection to cancer. International Journal of Cancer 131(10): 2349‑
- Segondy, Michel (2010) Papillomavirus et immunodépression 13.
- Muñoz N, Bosch FX, de Sanjosé S, Herrero R, Castellsagué X, et al. (2003) Epidemiologic classification of human papillomavirus types associated with cervical cancer. The New England Journal of Medicine 348(6): 518‑
- Rousseau M-C, Abrahamowicz M, Villa LL, Costa MC, Rohan TE, et al. (2003) Predictors of cervical coinfection with multiple human papillomavirus types. Cancer Epidemiology, Biomarkers & Prevention 12(10): 1029‑
- Jean-Luc P, Anne-Carole J, Xavier C, Jean-Francois C, Damian B, et al. (2008) Human papillomavirus (HPV) genotype distribution in invasive cervical cancers in France : EDITH 122(2): 428-432.
- Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S, et al. (2007) Human papillomavirus and cervical cancer. Lancet 370(9590): 890‑
- Veroux M, Corona D, Scalia G, Garozzo V, Gagliano M, et al (2009) Surveillance of human papilloma virus infection and cervical cancer in kidney transplant recipients : Preliminary data. Transplantation Proceedings 41(4): 1191‑
- Didelot-Rousseau M-N, Nagot N, Costes-Martineau V, Vallès X, Ouedraogo A, et al. (2006) Human papillomavirus genotype distribution and cervical squamous intraepithelial lesions among high-risk women with and without HIV-1 infection in Burkina Faso. British Journal of Cancer 95(3): 355‑
- Clifford GM, Gonçalves MAG, Franceschi S, (2006) Human papillomavirus types among women infected with HIV : A meta-analysis. AIDS (London, England) 20(18): 2337‑
- (2002) Recommandations pour la pratique clinique. Conduite à tenir devant une patiente ayant un frottis cervico-utérin anormal. ANAES.
- (2016) Conduite à tenir devant une femme ayant une cytologie cervico-utérine anormale. Recommandations et réfé INCa.
- Riethmuller D, Jacquard A-C, Lacau St Guily J, Aubin F, Carcopino X, et al. (2015) Potential impact of a nonavalent HPV vaccine on the occurrence of HPV-related diseases in France. BMC Public Health 15: 453.
- Sheikh S, Biundo E, Courcier S, Damm O, Launay O, et al. (2018) A report on the status of vaccination in Europe. Vaccine 36(33): 4979‑4992






























