Patient with Peripartum Cardiomyopathy Treated after 1 year of Optimal Medical Therapy, by CRT
Narcis Constantin Tăbăcaru1, Bogdan-Sorin Tudurachi1*, Alexandru Bostan1, Dora Diana Astratinei1, Radu A Sascău1,2 and Cristian Stătescu1,2*
1Prof Dr George IM Georgescu” Cardiovascular Diseases Institute, Romania
2Grigore T Popa” University of Medicine and Pharmacy, Romania
Submission: December 10, 2021; Published: December 14, 2021
*Corresponding author: Bogdan-Sorin Tudurachi, Prof Dr George IM Georgescu” Cardiovascular Diseases Institute, Romania
How to cite this article: Narcis C T, Bogdan-Sorin T, Alexandru B, Dora Diana A, Radu A S, et al. Patient with Peripartum Cardiomyopathy Treated after 1 year of Optimal Medical Therapy, by CRT. J Gynecol Women’s Health 2021: 22(5): 556096. DOI: 10.19080/JGWH.2021.22.556096
Abstract
Peripartum cardiomyopathy is a rare cause of heart failure in which left ventricular systolic dysfunction and symptoms of heart failure occur in young women in the last month of pregnancy or in the first months after delivery. Therapeutic management is similar to other forms of heart failure with low ejection fraction, and under optimal drug treatment most women will recover their left ventricular systolic function. A low percentage of patients will require the implantation of a cardiac resynchronization device or a left ventricular mechanical assistance device to improve systolic function.
We will present the case of a 38-year-old patient, with multiple pregnancy, diagnosed with peripartum cardiomyopathy in the last month of pregnancy, being characterized by severe impairment of systolic function of the left ventricle with an ejection fraction of 26% and left ventricular desynchrony secondary to the left bundle branch block. Under optimal medical therapy over 1 year no improvement in left ventricular systolic function was observed, so the patient was eligible for implantation of the cardiac resynchronization device. At six months of implantation of the device, normalization of the systolic function of the left ventricle was observed. Cardiac resynchronization therapy provides improvement in left ventricular systolic function in patients with peripartum cardiomyopathy in whom medical therapy has been ineffective.
Keywords: Peripartum cardiomyopathy; CRT; Heart failure; Left bundle branch block remodeling
Introduction
Peripartum cardiomyopathy is a rare cause of heart failure which according to the latest definition developed by the European Society of Heart Failure in 2010 is defined as an idiopathic cardiomyopathy characterized by heart failure secondary to systolic dysfunction of the left ventricle until the end of pregnancy or in the first few months after parturition in the absence any other cause of heart failure [1]. The overall incidence of peripartum cardiomyopathy varies by region, the highest being in African countries, meanwhile in developed countries the incidence varies between 1 case per 1000-4000 pregnancies [2]. From a clinical point of view, the majority cases are diagnosed after birth, the delay in establishing the diagnosis is mainly due to the overlap of the signs and symptoms of a normal pregnancy with those of heart failure [3].
Imagistically, echocardiography has an essential role in establishing the diagnosis of an LV systolic dysfunction: ejection fraction < 45% with or without LV dilation [4]. According to the European Heart Failure Guidelines, the treatment of peripartum cardiomyopathy is similar to other forms of heart failure with a low ejection fraction, but with an adjustment of the medication taking into account the harmful effects on the fetus [5]. If the left ventricular systolic function is severely impaired, cardiac defibrillator implantation therapies or cardiac resynchronization therapy may be considered. A very small number of patients require devices to assist left ventricular function or heart transplantation [1]. Thus, peripartum cardiomyopathy is a major cause of cardiac morbidity and mortality, but with a high probability of partial or complete recovery of LV systolic function [3].
Case Presentation
We present the case of a 38-year-old patient, with no cardiovascular history, without chronic medication at home, with an evolving pregnancy (34 weeks), addressed from a territorial hospital being diagnosed with acute heart failure. The patient was symptomatic of dyspnea at low exertion, progressively worsened in the last 2 weeks and elevated BP values at home (BP=160/90mmHg).
Both the hereditary collateral antecedents and the personal pathological antecedents were insignificant. From the personal medical history, we note that the patient was pregnant (pregnancy in progress 34 weeks), with 5 previous births, 4 healthy children, and a child who died 3 days after birth. The patient is a nonsmoker and denies alcohol and drugs consumptions.
Clinically, the patient was hemodynamically stable, conscious, with high blood pressure values (BP=160/90mmHg), tachycardia (HR=110/min), with a systolic murmur grade 2/6 in the mitral area, bilateral basal crackles, blood oxygen saturation level at around 93% SaO2. The laboratory findings: normocytic normochromic anemia (RBC=3.52 mil/mm3) Hemoglobin=11.3g/ dl, Hematocrit = 33.5% and increased natriuretic peptide values (NT-proBNP=2200pg/ml). Transthoracic echocardiography objectified a dilated left ventricle (EDV = 65mm; ESV = 310ml) with severe systolic dysfunction due to diffuse global hypokinesia, an estimated ejection fraction of 26%, with diastolic dysfunction type I. Associated with moderate functional mitral regurgitation, vena contracta = 6mm (Figure 1A).
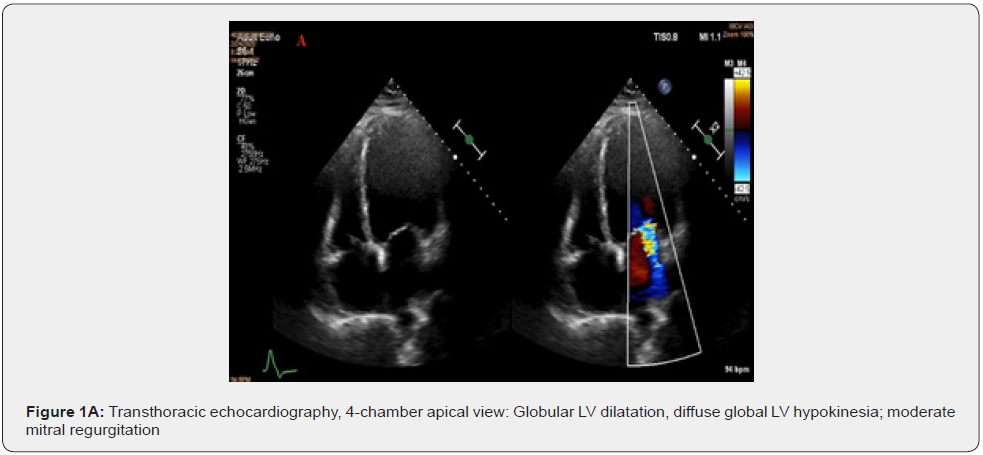
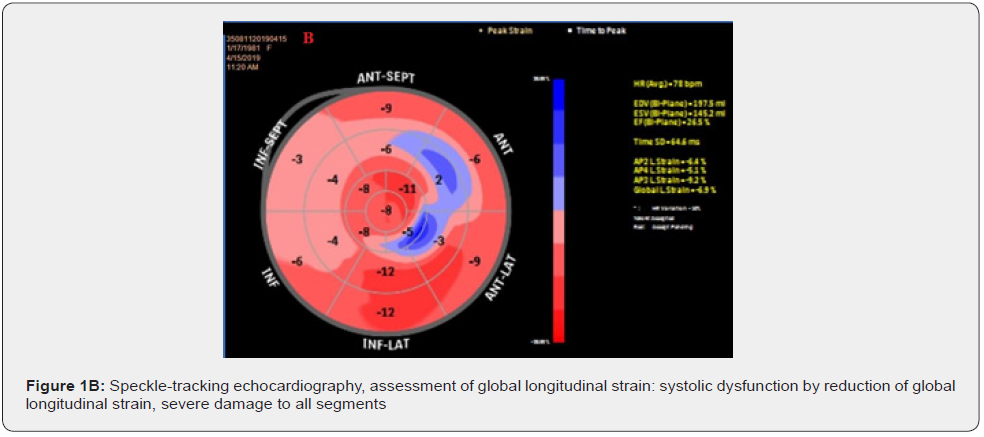
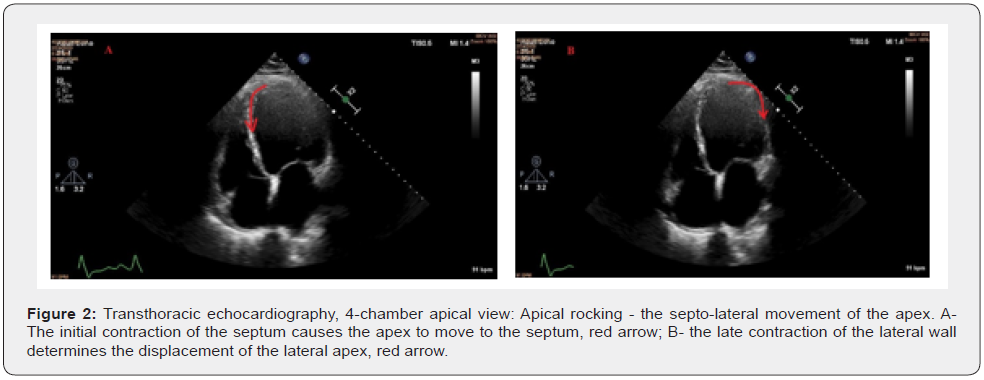
Assessment of global longitudinal strain of the left ventricle by speckle-tracking echocardiography identifies severe damage to all segments, GLS = -6.9% (Figure 1B). In addition to the electrical asynchrony objectified by the presence of left bundle branch block on the electrocardiogram, the echocardiographic examination reveals apical rocking (septo-lateral displacement of the apex during ventricular systole) visual parameter used to assess mechanical dyssynchrony (Figure 2A&2B).
During hospitalization, on day 2, the patient’s condition worsens, she develops an episode of acute pulmonary edema. After stabilizing the patient by using non-invasive ventilation, volume depletion through intravenous administration of loop diuretic and intravenous nitroglycerin, the multidisciplinary team consisting of cardiologist, gynecologist, neonatologist, anesthesia and intensive care physician and cardiovascular surgeon decides to perform an emergency cesarean section. Subsequently, the evolution of the patient and the newborn is favorable.
Therapeutic management after birth consisted of hygienicdietary indications, the main measure being ablactation and specific drug treatment for moderate forms of the disease according to the acronym BOARD [3]:
B: Bromocriptine 2.5mg 1cpx2 / day for 2 weeks, then 1cp / day for 6 weeks.
O: Oral therapy specific for heart failure: Sacubitril / Valsartan 24/26 mg 1cpx2 / day; Carvedilol 3,125mg 1 cpx2 / day; Spironolactone 25mg 1cp / day
A: Anticoagulant: due to the risk of deep vein thrombosis during treatment with bromocriptine Enoxaparin 0.6 ml / day in prophylactic dose
R: Vasorelaxant drug: nitroglycerin given intravenously during the acute phase
D: Diuretic therapy: Furosemide 40mg 1cp / day
At the 1-year reevaluation the patient showed a slight improvement in exercise capacity and the echocardiographic evaluation showed a slight improvement in left ventricular ejection fraction (LVEF = 30%). Due to the persistence of an LV systolic dysfunction in the presence of a left bundle branch block, it is decided to perform coronary angiography to exclude an ischemic etiology. The investigation finds normal coronary arteries.
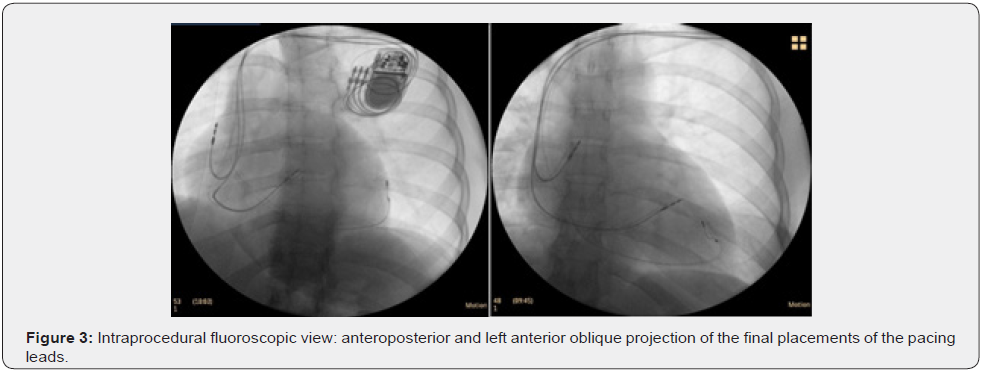
Given the following parameters: NYHA II functional class, the presence of a LBBB with a duration of the QRS complex of 160 ms on the electrocardiogram and severe LV systolic dysfunction with LVEF 30% under optimal drug therapy, it is decided to implant un cardiac resynchronization device (CRT-P). (Figure 3). 6 months after the implantation of the cardiac resynchronization device, a significant improvement was observed both from a clinical point of view - asymptomatic patient to intense physical exertion, and imagistically - complete recovery of LV systolic function (Figure 4).

Discussion
The presence of acute heart failure in a young patient, with no other cardiovascular history, with an evolving pregnancy, is a challenge both in terms of diagnosis and especially in terms of therapeutical management. In the third trimester of pregnancy the main etiologies of acute heart failure are preeclampsia and peripartum cardiomyopathy [1]. The diagnosis of peripartum cardiomyopathy is a diagnosis of exclusion. According to the latest guide of the European Society of Cardiology for the diagnosis and management of peripartum cardiomyopathy in 2019, the diagnosis is established based on the following parameters: the presence of risk factors (African American race, multiple pregnancy, multiparity, age over 30, preeclampsia and pre-existent hypertension); onset of symptoms at the end of pregnancy or in the first months after birth; biologically significantly elevated values of the natriuretic peptide NT-proBNP, and echocardiographic systolic dysfunction of the left ventricle with LVEF <45% in the absence of other causes of heart failure (congenital malformations, hypertrophic cardiomyopathy, valvulopathy, chemotherapy, etc.) [3].
Peripartum cardiomyopathy is a rare cause of heart failure, but with a high morbidity and mortality rate. Estimated mortality differs by race, geographical region and duration of follow-up. In the long term, mortality at 5 years varies between 7% and 20%, with high values in African American patients and those in African countries [6]. Peripartum cardiomyopathy is associated with a high rate of recovery of cardiac function, between 50-80% of patients will fully recover their heart function in the first 12 months [7]. Late recovery may occur in a small number of cases even 2 years after diagnosis [8].
The IPAC (Investigations of Pregnancy Associated Cardiomyopathy) study conducted in America, which included 100 women (30% African American) diagnosed with peripartum cardiomyopathy, observed for a period of 12 months, reveled a complete recovery of heart function in 72% of cases [9]. A low recovery rate (45%) was reported in a study of 55 patients (51% African American patients) after a mean follow-up of 29 months [6]. Numerous predictive factors have been studied, among them the most important prognostic factor for the risk of adverse effects and long-term survival is the ejection fraction at the time of diagnosis [10]. Patients with severe LV systolic dysfunction (LVEF < 30%) have a smaller percentage of complete or partial recovery of systolic function.
In the IPAC study, the patients with LVEF < 30%, 66% partially recovered or had worsening systolic dysfunction under optimal drug treatment. In a sub-analysis of the IPAC study, in patients with LVEF < 30% and severe LV dilation (EDV ≥ 60 mm) it was observed that there was no recovery of systolic function after 12 months under optimal drug treatment [9]. Therapeutic management of patients with peripartum cardiomyopathy is similar to that of other forms of chronic heart failure with a low ejection fraction. The etiology of peripartum cardiomyopathy is still unclear, but one of the pathogenic mechanisms is the hypersecretion of the hormone prolactin which is cleaved into small fragments (16KDa prolactin) which will cause endothelial dysfunction and subsequent damage to cardiomyocytes with a continued inflammatory response in the ventricular myocardium.
A therapy for blocking this pathogenic mechanism is bromocriptine, a dopaminergic agonist that inhibits prolactin formation. The use of this new treatment option in patients with peripartum cardiomyopathy is based on data from a randomized multicenter study that included 63 patients with peripartum cardiomyopathy with LVEF < 35% who received bromocriptine for 8 weeks in standard heart failure therapy. After a period of 6 months, 68% of patients showed complete recovery of the ejection fraction assessed by MRI [11].
Less than 5% of patients with peripartum cardiomyopathy have left bundle branch block [12]. In the presence of this conduction disorder, the implantation of the cardiac resynchronization device should be considered [13].
The patient presents all the criteria for the implantation of the cardiac resynchronization device: LBBB with QRS duration of 160 ms, persistence of systolic LV dysfunction (LVEF = 30%) under optimal drug treatment. Also, female gender, the nonischemic etiology of dilated cardiomyopathy are major predictors of CRT response. In the category of patients responding to CRT, a subcategory called super-responses are characterized by normalized systolic function (LVEF ≥ 50%) is described. The percentage of super-responsive patients varies between 10 - 30% according to clinical studies [13]. There are no clinical studies evaluating the percentage of patients with peripartum cardiomyopathy who respond to CRT, and only a small number of cases have been published in the literature, most reporting a significant improvement in systolic function after CRT [12,14].
Thus, according to data from large randomized clinical trials evaluating the benefit of CRT in patients with chronic heart failure with a significantly lowered ejection fraction (LVEF ≤ 35%) and the presence of ECG conduction disorders with QRS duration over > 120ms, approximately 70% of patients will respond to CRT with a significant improvement in functional capacity and increased long-term survival [15-18].
Conclusion
Peripartum cardiomyopathy is a rare cause of heart failure, but with high mortality in the absence of specific heart failure therapy. The clinical picture is similar to other forms of non-ischemic dilated cardiomyopathy, but the absence of a history of heart damage and the onset of symptoms in either late pregnancy or the first months after birth are suggestive of the diagnosis. Echocardiography is the main imaging method for establishing the etiological diagnosis, modern imaging methods such as cardiac MRI are used to assess the prognosis, late gadolinium enhancement being associated with a negative long-term prognosis.
A relatively high percentage of cases with severe LV systolic dysfunction do not respond to optimal drug treatment. For this category of patients, in the presence of LBBB with a QRS complex duration of more than 120 ms, the opportunity to implant the cardiac resynchronization device should be evaluated. This interventional therapy in patients with peripartum cardiomyopathy improves the quality of life of the patient as well as the long-term survival rate.
References
- Davis M, Arany Z, McNamara D, Goland S, Elkayam U (2020) Peripartum Cardiomyopathy: JACC State-of-the-Art Review. J Am Coll Cardiol 75(2): 207-221.
- Kolte D, Khera S, Aronow W, Palaniswamy C, Mujib M, et al. (2014) Temporal Trends in Incidence and Outcomes of Peripartum Cardiomyopathy in the United States: A Nationwide Population‐Based Study. J Am Heart Assoc 3(3): e001056.
- Bauersachs J, König T, Meer P, Petrie M, Hilfiker‐Kleiner D, et al. (2019) Pathophysiology, diagnosis and management of peripartum cardiomyopathy: a position statement from the Heart Failure Association of the European Society of Cardiology Study Group on peripartum cardiomyopathy. Eur J Heart Fail 21(7): 827-843.
- Regitz-Zagrosek V, Roos-Hesselink J, Bauersachs J, Blomström-Lundqvist C, Cífková R, et al. (2018) 2018 ESC Guidelines for the management of cardiovascular diseases during pregnancy. Eur Heart J 39(34): 3165-3241.
- Ponikowski P, Voors A, Anker S, Bueno H, Cleland J, et al. (2016) 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 37(27): 2129-2200.
- Mahowald M, Basu N, Subramaniam L, Scott R, Davis M, et al. (2019) Long-term Outcomes in Peripartum Cardiomyopathy. The Open Cardiovascular Medicine Journal 13(1): 13-23.
- Sliwa K, Petrie M, Hilfiker‐Kleiner D, Mebazaa A, Jackson A, et al. (2018) Long‐term prognosis, subsequent pregnancy, contraception and overall management of peripartum cardiomyopathy: practical guidance paper from the Heart Failure Association of the European Society of Cardiology Study Group on Peripartum Cardiomyopathy. Eur J Heart Fail 20(6): 951-962.
- Pillarisetti J, Kondur A, Alani A, Reddy M, Reddy M, et al. (2014) Peripartum Cardiomyopathy. Journal of the American College of Cardiology 63(25): 2831-2839.
- McNamara D, Elkayam U, Alharethi R, Damp J, Hsich E, et al. (2015) Clinical Outcomes for Peripartum Cardiomyopathy in North America: Results of the IPAC Study (Investigations of Pregnancy-Associated Cardiomyopathy). J Am Coll Cardiol 66(8): 905-914.
- Goland S, Bitar F, Modi K, Safirstein J, Ro A, et al. (2011) Evaluation of the Clinical Relevance of Baseline Left Ventricular Ejection Fraction as a Predictor of Recovery or Persistence of Severe Dysfunction in Women in the United States With Peripartum Cardiomyopathy. Journal of Cardiac Failure 17(5): 426-430.
- Hilfiker-Kleiner D, Haghikia A, Berliner D, Vogel-Claussen J, Schwab J, et al. (2017) Bromocriptine for the treatment of peripartum cardiomyopathy: a multicentre randomized study. Eur Heart J 38(35): 2671-2679.
- Richard E, Turgeon P, Dubois M, Sénéchal M (2019) Early Use of Cardiac Resynchronization Therapy to Accelerate Symptomatic Relief and Complete Left Ventricular Function Recovery in Peripartum Cardiomyopathy. Medicina (Kaunas) 55(6): 246.
- Normand C, Linde C, Singh J, Dickstein K (2018) Indications for Cardiac Resynchronization Therapy. JACC: Heart Failure 6(4): 308-316.
- Mouquet F, Mostefa Kara M, Lamblin N, Coulon C, Langlois S, et al. (2012) Unexpected and rapid recovery of left ventricular function in patients with peripartum cardiomyopathy: impact of cardiac resynchronization therapy. Eur J Heart Fail 14(5): 526-529.
- Cleland J, Daubert J, Erdmann E, Freemantle N, Gras D, et al. (2005) The Effect of Cardiac Resynchronization on Morbidity and Mortality in Heart Failure. N Engl J Med 352(15): 1539-1549.
- Wang N, Jain S (2021) Peripartum cardiomyopathy and cardiac resynchronization therapy: Case reports and literature review. HeartRhythm Case Rep 7(11): 767-772.
- Moss A, Hall W, Cannom D, Klein H, Brown M, et al. (2009) Cardiac-Resynchronization Therapy for the Prevention of Heart-Failure Events. N Engl J Med 361(14): 1329-1338.
- Tang A, Wells G, Talajic M, Arnold M, Sheldon R, et al. (2010) Cardiac-Resynchronization Therapy for Mild-to-Moderate Heart Failure. N Engl J Med 363(25): 2385-2395.






























