The Role of Recurrent Vulvovaginal Candidiasis and Comorbidities in the Etiology of Vestibulodynia: Data from the Vunet Study
Alessandra Graziottin1,3*, Filippo Murina2, Dania Gambini3 and Elena Boero3
1Center of Gynecology and Medical Sexology H San Raffaele Resnati Milan, Italy
2Lower Genital Tract Disease Unit, V Buzzi Hospital-University of the Study of Milan, Italy
3Alessandra Graziottin Foundation for the cure and care of pain in women - NPO, Italy
Submission: October 15, 2021; Published: October 22, 2021
*Corresponding author: Alessandra Graziottin, Center of Gynecology and Medical Sexology H San Raffaele Resnati Milan, Alessandra Graziottin Foundation for the cure and care of pain in women – NPO, Italy
How to cite this article: Alessandra G, Filippo M, Dania G, Elena B. The Role of Recurrent Vulvovaginal Candidiasis and Comorbidities in the Etiology of Vestibulodynia: Data from the Vunet Study. J Gynecol Women’s Health 2021: 22(3): 556087. DOI: 10.19080/JGWH.2021.22.556087
Abstract
Women with provoked vestibulodynia (PVD) are more likely to report a history of vulvovaginal candidiasis (VVC) and recurrent VVC (RVVC). An observational Italian study was undertaken to analyze how many women with PVD have an RVVC as a leading trigger and to describe predisposing factors. The cross-sectional-based study, named VuNet (Vulvodynia Network), was performed among consecutive female patients affected by chronic vulvar pain (symptoms lasting at least 3 months) attending 21 Italian medical centers (public hospital, university clinic, and private outpatient services). A total of 1183 subjects with a diagnosis of chronic vulvar pain were included in the study, presenting leading symptoms such as vulvar pain (90%), vulvar burning (97%), and pain during and after intercourse (96%). 70,8% of women were diagnosed with spontaneous or provoked PVD and 27,3% with generalized vulvodynia. RVVC was complained of by 32% of patients. Family history showed a higher prevalence of diabetes mellitus (mother=8,4%, father=8,6%), compared to the prevalence in the Italian population <65 years (5,3%). Clinical examinations found a hyperactive pelvic floor in 71, 6%. The recurrent/persistent inflammation associated with an aberrant immune-allergic reaction to Candida antigens may be a strong co-factor for developing PVD in this cluster of patients. Our data highlight the importance of investigating metabolic vulnerabilities to diabetes, both in the patient and her family; check for a hyperactive pelvic floor, predisposing to introital microabrasions, and encourage appropriate lifestyles, including reducing the consumption of glucose or saccharose, controlling body weight, and performing daily aerobic exercise to reduce peripheral insulin resistance. The ultimate goals are reducing predisposing factors for candidiasis, PVD, and coital pain in this cluster of patients, and tailoring treatment accordingly.
Keywords: Vulvar pain; Vulvar burning; Dyspareunia; Coital pain; Diabetes; Hyperactive pelvic floor
Abbreviations: PVD: Provoked Vestibulodynia; RVVC: Recurrent Vulvovaginal Candidiasis; VVC: Vulvovaginal Candidiasis; VVS: Vulvar Vestibulitis Syndrome
Introduction
Provoked vestibulodynia (PVD) is characterized by tenderness or pressure in the vulvar vestibule of at least 3 months duration [1,2]. It is estimated to affect more than one-tenth of women in their lifetime [3,4]. Typically, pain is provoked by sexual intercourse, the use of tampons, and tight clothing. The etiology of PVD is still controversial; it is generally thought to be multifactorial, with several pathophysiological factors involved, including inflammatory, immune, hormonal, congenital, genetic, neuroproliferative, and muscular [5,6]. PVD is characterized by the progressive shift of pain from nociceptive, a signal of acute tissue damage, to chronic/inflammatory, discouraging contact with the interested area, and finally to neuropathic or dysfunctional, when pain happens in the absence of noxious stimuli and becomes a prominent disease per se [2]. This view is confirmed by the many histological features suggesting an initial inflammatory state of the vulvar tissue, such as a significant increase of mast cells and high concentration of degranulation products, including heparanase,which have been correlated to vulvodynia in multiple works [7- 11]. Mast cells products might induce tissue hyperinnervation, possibly explaining neuropathic pain in the later stages of the disease [9]. Despite this evidence, the current definition of PVD does not include anymore the phrase “Vulvar Vestibulitis Syndrome” (VVS), thus not including the inflammatory features typical of the early phases of the vestibular inflammation [12,13]. In the context of this work, we believe that the pathophysiology of vestibular pain could be more appropriately described when both terms are maintained (VVS/PVD), like in a two-times movie. In the first time, the pathophysiology is prominently inflammatory, in the second time is progressively dominated by pain. This reading is particularly appropriate when considering the role of recurrent yeast infections as a prominent inflammatory trigger in the etiology of VVS/PVD, at least in the cluster of patients described in this work [14].
Vulvovaginal candidiasis (VVC) is caused by abnormal growth of Candida spp., typically Candida albicans, on the female genital tract mucosa [15]. The infection affects 70–75% of women at least once during their lives, most frequently young women of childbearing age, and 40–50% of women will experience a recurrence [16]. It is estimated that 6-9% of adult women have recurrent VVC (RVVC), defined as four or more episodes every year [17]. Diagnosis is not straightforward, as VVC is defined by the combination of often non-specific vaginal symptoms and the presence of yeast, which is a common vaginal commensal. Estimating the incidence and prevalence is challenging: most VVC is diagnosed and treated empirically, the availability for purchase of effective therapies over the counter enables self-diagnosis and treatment, and the duration of the relatively benign VVC symptoms is short, introducing errors into any estimates relying on medical records or patient recall [17].
Women with PVD are more likely to report a history of VVC, especially RVVC [18-20], suggesting that a susceptibility to Candida infection and related comorbidities, coupled with reduced capacity to terminate the resulting inflammation, could initiate an inflammatory cascade that triggers neurogenic changes in the vestibule, leading to a persistently altered pain response [5]. The challenge related to RVVC is to prevent both the recurrences and to avoid PVD, which is reported to affect 8,6-10% of women [21,22]. Some criticisms are a subject under discussion: how many women with PVD have an RVVC as a leading trigger of this pathology? What are the predisposing, precipitating, and maintaining factors? What are the most prominent comorbidities?
In this work, we present the relevant data from an observational national study undertaken in Italy to address these relevant clinical questions. Here we focus on the relationship between RVVC and VVS/PVD, with special attention to clustered predisposing factors. A deeper insight on RVVC will be presented, to read the data of the current study with a more comprehensive vision of the pathophysiology behind the association.
Materials and Methods
A cross-sectional based study, named VuNet (Vulvodynia Network), was performed among consecutive female patients affected by chronic vulvar pain (symptoms lasting at least three months) attending to 21 Italian medical centers (public hospital, university clinic, and private outpatient services) spread over the national territory, during December 2016 to November 2018. The VuNet project was finalized to draw the attention of physicians, public opinion/media, and institutions to the neglected problem of vulvar pain. The pillars of the project were research and training.
At the first visit, each health care professional (gynecologists, pelvic floor rehabilitation therapists, sexologists, and urologists) involved in the study entered the data in a special web-based medical record (PRIDE- Progetto Rete Italiana Dolore vulvarE) designed by the Authors, regarding epidemiological aspects, demographic characteristics, obstetric and gynecological history, presence and duration of current or past symptoms, associated disorders, physical examination and approach to treatment. Women who agreed to participate in the study were included after giving vis-a-vis informed consent at the start of the personal visit and medical online data entry.
Descriptive statistics were performed for each measured variable: mean values with standard deviations were calculated for continuous measures and frequencies or percentages for categorical measures. Associations between quantitative variables with two categories were explored using the student t-test for independent samples in the case of normal data distribution and using the McNemar test for dichotomous parameters. Statistical significance was considered with p < 0.05 and statistical analysis was performed using the SPSS Statistical Package (version 20.0, SPSS, Armonk, NY, USA).
Result and Discussion
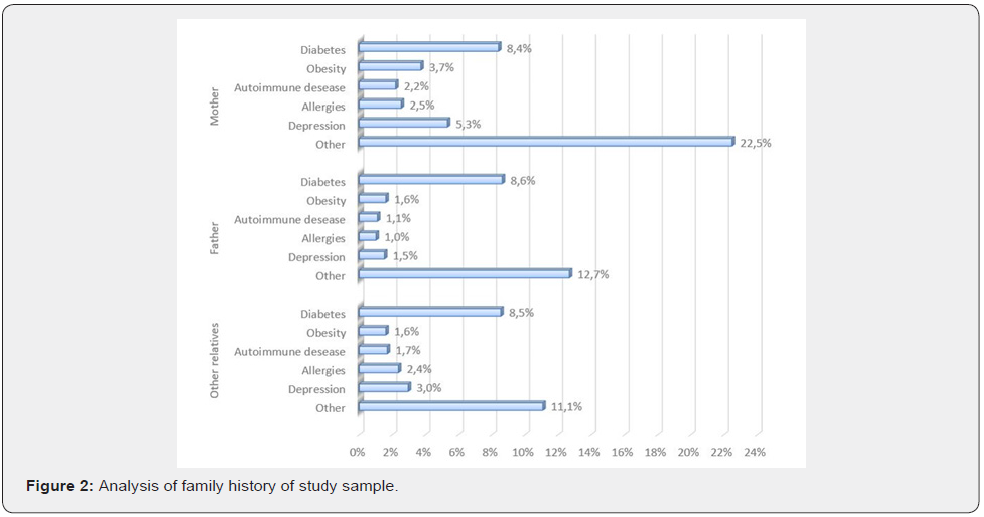
A total of 1183 subjects with a diagnosis of chronic vulvar pain (symptoms lasting at least 3 months) were included in the study. The demographics and baseline characteristics of the participants are shown in Table 1. The mean age was 40.3±13.3 years (range 13- 79) and the most common age group was 20-49 years, comprising 70.7% of subjects. Motivations to the consultation are reported in Figure 1. Coital pain/dyspareunia was the leading complaint in 64% of women; vaginal dryness was second, with 29.8% of women reporting it as a most bothersome symptom; 24% complain of vulvar pain, either spontaneous or elicited by intercourse; 17% had an active VVC at the moment of the consultation. The analysis of familiar history revealed a relatively higher presence of diabetes mellitus (mother=8,4% and father=8,6, %) (Figure 2).
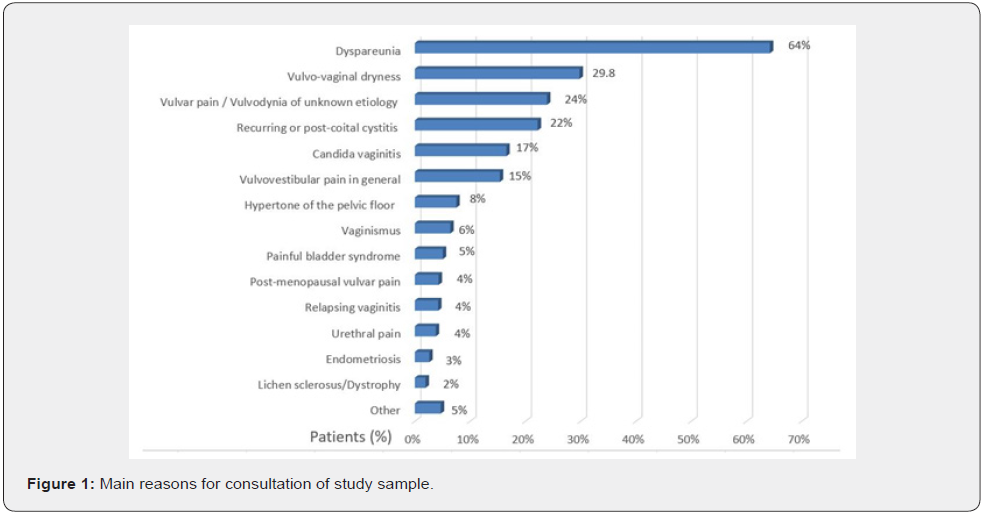
Quality data emerge from women’s wording: “Intercourse triggers the candidiasis”, “Intercourse triggers/worsens the burning pain at the entrance of the vagina”; “The feeling is that it causes tiny cutting (“micro-abrasions”) there”. Of note, RVVC was complained of by 32% of patients (Figure 3). High comorbidity with recurrent cystitis (19, 5%) and post-coital cystitis (17, 9) % was reported. Overall, 37, 4 % of our patients with vulvar pain have a history of recurrent cystitis and multiple antibiotic treatments. At the physical examination, 71, 6% were diagnosed with a hyperactive pelvic floor. The clinical diagnosis had a leading 70, 8% matching the criteria for spontaneous or provoked PVD and 27, 3% of generalized vulvodynia, with a comprehensive diagnosis of vulvar pain in 98,1% of consulting patients.
Several practical considerations emerge from the present analysis of the VuNet data, focused on RVVC in women with VVS/ PVD. First and foremost, our survey highlights a significantly higher prevalence of diabetes in the family of women suffering from PVD (8,4% in mothers, 8,6% in fathers), compared to the Italian population (5.3%) in 2016 [23]. To the authors’ knowledge, this is the first time such an important risk factor is heightened in research on VVS/PVD. Familiarity for diabetes may suggest a genetic predisposition of patients that increases their risk of having an inadequate glucose metabolism, higher peripheral resistance to insulin, more persistent higher levels of glycemia, i.e., in the higher quartile of the norm or above, and increased risk of VVC [15,24-26].
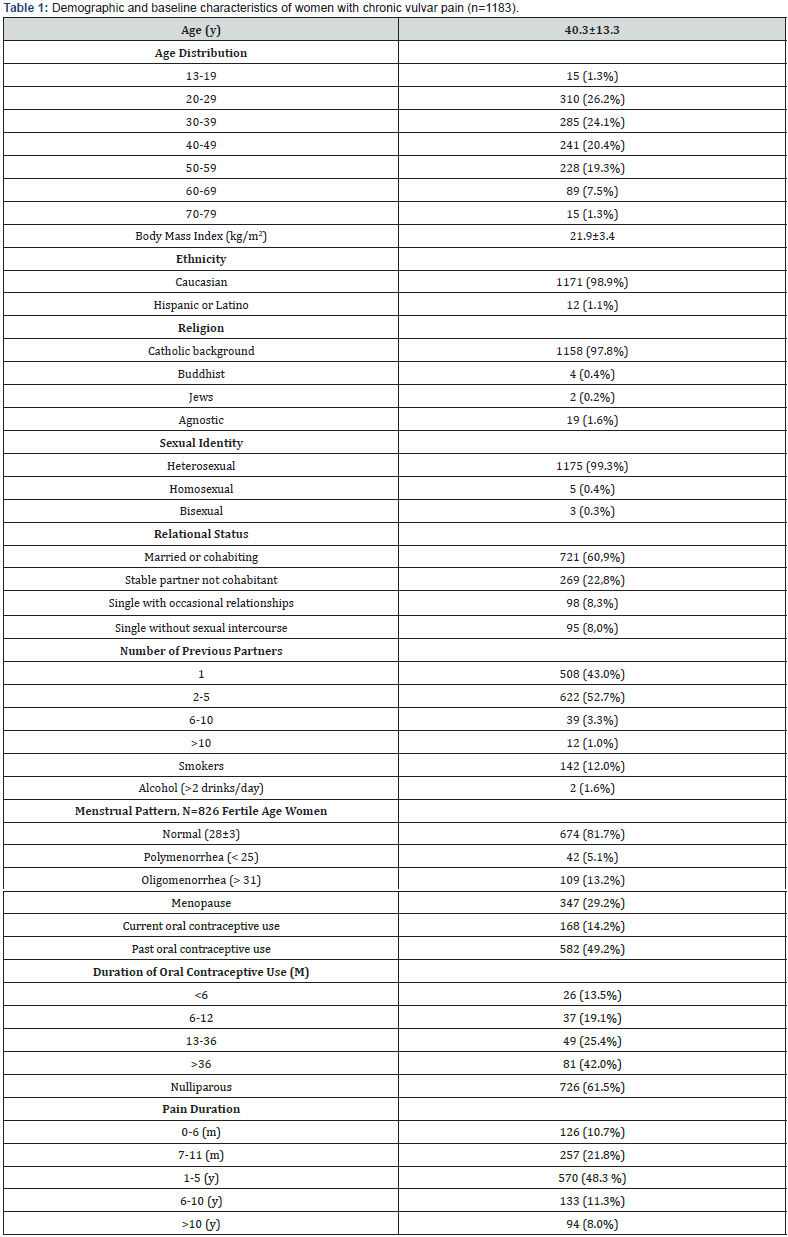
Data reported as number and percentages (%) or mean ± standard deviation. y=years; m=months
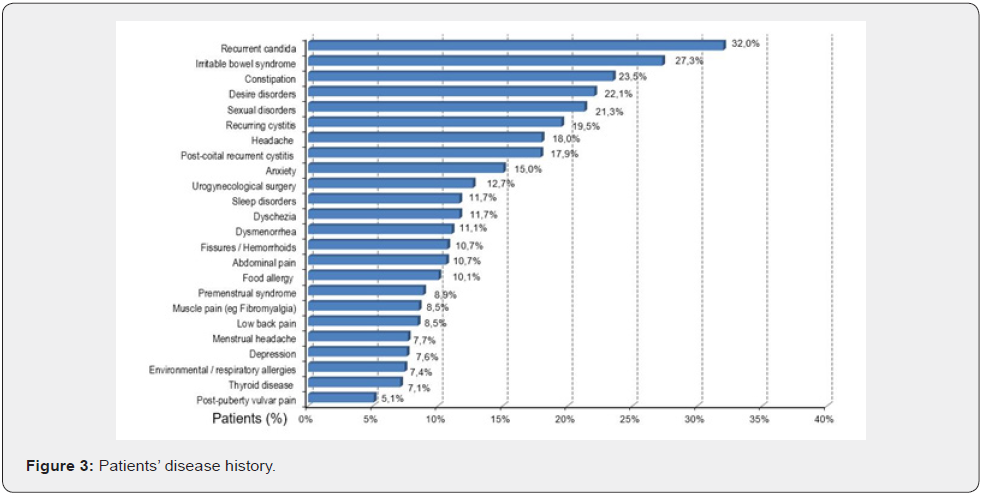
The second strength of our study is to prove that more than one-third of our patients with VVS/PVD has a history or current complaint of RVVC (32%) [27], a percentage significantly higher than the figures currently reported for the general population [23]. Healthcare providers struggle to identify criteria to predict which patients with RVVC will initiate a more challenging and chronic PVD. The link between the previous Candida infections and the higher risk of long-term consequences is probably explained by an aberrant hyperactive immuno-allergic response, likely to stem from a genetic vulnerability to allergies, inflammation, and autoimmune diseases [20,28,29]. This hypothesis of an aberrant hyperactive immunologic response may be supported by the high percentage of our patients suffering from allergies (17, 5%): food allergies, 10,1%, respiratory allergies 7,4% [27].
Several studies and clinical practice support this association (10). A rodent model of long-lasting mechanical allodynia, after repeated exposure of the vulva to Candida albicans, has been developed. The study mimics repeated vaginal candidiasis that some women report when they experience VVS/PVD. In the rodent model allodynia persists for at least three weeks after resolution of infection [30]. In a subset of female mice, in this very same model, it produces hyperinnervation [30]. Selective sampling of fibroblasts from painful and adjacent nonpainful sites demonstrates enhanced pro-inflammatory cytokine production after stimulation with yeast extract [31], another aspect that supports the maintenance of an inflammatory etiology, contributing to the onset and the persistence of vestibular pain.
As mentioned above, a susceptibility to Candida infection and related comorbidities is coupled with a genetic background indicating a reduced capacity to terminate the inflammation and reduced capacity to terminate Candida albicans infections [32]. A polymorphism in the gene coding for mannose-binding lectin, an innate immune antimicrobial protein that inhibits Candida proliferation, and polymorphisms in the genes coding for inflammasomes (macromolecules that regulate the release of interleukin IL-1ß) reduce the production of active IL-1ß necessary for recruitment of immune cells that inactivate yeast, are more common in VVS/PVD patients with RVVC [32]. Candida albicans initially interacts with epithelial cells, resulting in fungal recognition and the formation of hyphae. Hyphae formation is critical for host cell damage and immune activation, which are both driven by the secretion of candidalysin, a recently discovered peptide toxin [33]. Epithelial activation leads to the production of inflammatory mediators that recruit innate immune cells including neutrophils, macrophages, inducing innate Type 17 responses, which together work with epithelial cells to clear the fungal infection [34]. A growing body of evidence supports the leading pathogenetic role of candidalysin in triggering all the cascade of inflammatory events behind the symptoms reported by patients [35]. The mechanisms described may induce a persistent inflammation of the vestibule, with the typical triad of increased concentration of mast cells, degranulated mast cells, and mast cells closer to the pain fibers [1,22], with a resultant proliferation and condensation of pain fibers [1, 22]. This is the histologic feature associated with the complaint of hyperalgesia and later, burning pain at touch contact (allodynia), when the proliferated pain fibers cross the basal membrane of the vestibular mucosa along the tunnels created by the heparanase and tryptase enzymes secreted by the hyperactivated mast cells.
The third relevant clinical finding is the high percentage (71,6%) of women diagnosed with a hyperactive pelvic floor, either lifelong or acquired [27]. Vestibular pain may contribute to the defensive contraction of the elevator ani muscle that further squeezes the vaginal entrance, mechanically predisposing to microlesions of the introital mucosa at penetration and at thrusting during intercourse. Introital pain further activates an inhibitory reflex on vaginal lubrication, thus causing/worsening vaginal dryness. This increases the risk of introital microabrasions, facilitating the exposure of the hyperactive immune system to vaginal Candida spp [20].
Another finding is the high comorbidity with recurrent cystitis (19, 5%) and post-coital cystitis (17,9%). Overall, 37, 4% of our patients with vulvar pain have a history of recurrent cystitis and multiple antibiotic treatments. Antibiotics alter the intestinal and vaginal microbiota, stimulating an overgrowth of bowel and vaginal Candida spp., concur in bowel inflammation and predispose to more aggressive recurrent candidiasis, contributing to RVVC [15,36,37]. Worth of note, the hyperactive pelvic floor contributes as well to the biomechanical etiology of recurrent post-coital cystitis reported by these patients.
Conclusion
Overall, the VuNet study contributes to a more comprehensive reading of predisposing, precipitating, and maintaining factors that contribute to vulvar pain. It helps clinicians recognizing clusters of patients that present with RVVC as a predisposing factor contributing to vestibular inflammation. This cluster often presents with diabetic familiarity, suggesting a vulnerability to recurrent Candida infections due to poor glycemic control; allergies, which may suggest a predisposition to aberrant inflammatory responses to Candida antigens in the vulva. Finally, these patients often display a hyperactive pelvic floor, a common denominator of both introital dyspareunia/vestibular microlesions during intercourse/RCCV and post-coital cystitis. They deserve a more tailored, individualized multimodal approach, with a special focus on RVVC, persistent vestibular inflammation, and hyperactive pelvic floor. Looking carefully for women with a history of RVVC and VVS/PVD, the physician may offer a more pathophysiologicalbased treatment, with a higher rate of success.
Acknowledgment
The authors thank the members of the VuNet Study Group: S. Taraborrelli (Bologna), B. Gardella (Pavia), M. Campo (Ragusa), R.Cirillo (Genova), P. Salzano (Napoli), A. Bortolami (Padova), N. Russo (Roma), M. Sansone (Napoli), B. Dionisi (Roma), F.Fruzzetti (Pisa), C. Nanini (Pisa), T. Bisanti (Lecce), B. Del Bravo (Pisa), D. Grassi (Modena), A. Criscuolo (Roma), R. Papeo (Bari), F. La Mantia (Palermo), B. Landi (Ancona), C. Mainini (Napoli), C. Polo (Brescia), G. Cognini (Bologna).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ethical Statement
The research office at the authors’ institution reviewed the study and determined that the study does not require human research protection oversight by the Institutional Review Board. Furthermore, the study complied with the European regulations on clinical studies, and with the international ethical recommendations on research.
Author Contribution
AG, FM, and DG designed the research study, performed the research, and analyzed the data, and wrote the manuscript. EB contributed to the text writing, editing, and revision.
Funding
The project was conceived by the Authors, AG, and FM, and it was supported by the Alessandra Graziottin Foundation for the cure and care of pain in women, NPO, and by the Italian Association of Vulvodynia (AIV) NPO.
References
- Pukall CF, Goldstein AT, Bergeron S, David F, Stein A, et al. (2016) Vulvodynia: Definition, Prevalence, Impact, and Pathophysiological Factors. J Sex Med 13(3): 291-304.
- Henzell H, Berzins K, Langford JP (2017) Provoked vestibulodynia: current perspectives. Int J Womens Health 9: 631-642.
- Reed BD, Harlow SD, Sen A, Legocki LJ, Edwards RM, et al. (2012) Prevalence and demographic characteristics of vulvodynia in a population-based sample. Am J Obstet Gynecol 206(2): 170.e1-170.e9.
- Vieira-Baptista P, Lima-Silva J, Cavaco-Gomes J, Beires J (2014) Prevalence of vulvodynia and risk factors for the condition in Portugal. Int J Gynecol Obstet 127(3): 283-287.
- Stockdale CK, Lawson HW (2014) 2013 Vulvodynia guideline update. J Low Genit Tract Dis 18(2): 93-100.
- Murina F, Graziottin A, Felice R, Di Francesco S (2016) Coital pain in the elderly: could a low dose estriol gel thrill the vulvar vestibule? Eur J Obstet Gynecol Reprod Biol 207: 121-124.
- Bohm-Starke N, Hilliges M, Falconer C, Rylander E (1998) Increased intraepithelial innervation in women with vulvar vestibulitis syndrome. Gynecol Obstet Invest 46(4): 256-260.
- Bohm-Starke N, Hilliges M, Falconer C, Rylander E (1999) Neurochemical characterization of the vestibular nerves in women with vulvar vestibulitis syndrome. Gynecol Obstet Invest 48(4): 270-275.
- Bornstein J, Cohen Y, Zarfati D, Shifra S, Ella O, et al. (2008) Involvement of heparanase in the pathogenesis of localized vulvodynia. Int J Gynecol Pathol 27(1): 136-141.
- Bornstein J, Goldschmid N, Sabo E (2004) Hyperinnervation and mast cell activation may be used as histopathologic diagnostic criteria for vulvar vestibulitis. Gynecol Obstet Invest 58(3): 171-178.
- Halperin R, Zehavi S, Vaknin Z, Ben-Ami I, Pansky M, et al. (2005) The major histopathologic characteristics in the vulvar vestibulitis syndrome. Gynecol Obstet Invest 59(2): 75-79.
- Bohm-Starke N (2010) Medical and physical predictors of localized provoked vulvodynia. Acta Obstet Gynecol Scand 89(12): 1504-1510.
- Bornstein J, Goldstein AT, Stockdale CK, Bergeron S, Pukall C, et al. (2016) 2015 ISSVD, ISSWSH and IPPS consensus terminology and classification of persistent vulvar pain and vulvodynia. Obstet Gynecol 127(4): 745–751.
- Graziottin A, Gambini D, Bertolasi L (2015) Genital and sexual pain in women. Handb Clin Neurol 130: 395-412.
- Gonçalves B, Ferreira C, Alves CT, Mariana H, Azeredo J, et al. (2016) Vulvovaginal candidiasis: Epidemiology, microbiology and risk factors. Crit Rev Microbiol 42(6): 905-927.
- Sobel JD (2007) Vulvovaginal candidosis. Lancet 369(9577): 1961-1971.
- Sobel JD (2016) Recurrent vulvovaginal candidiasis. Am J Obstet Gynecol 214(1): 15-21.
- https://www.acog.org/clinical/clinical-guidance/committee-opinion/articles/2016/09/persistent-vulvar-pain
- Sobel JD (1997) Vaginitis. N Engl J Med 337(26): 1896-1903.
- Ramirez DKHM, McCormick TS, Do SO, Wendy G, Mahmoud AG, et al. (2005) Cutaneous hypersensitivity to Candida albicans in idiopathic vulvodynia. Contact Dermatitis 53(4): 214-218.
- Leusink P, Pasch VDS, Teunissen D, LaanET, Lagro-Janssen AL, et al. (2018) The relationship between vulvovaginal candidiasis and provoked vulvodynia: A systematic review. J Sex Med 15(9): 1310-1321.
- Graziottin A, Murina F (2017) Vulvar Pain: From Childhood to Old Age. In: (1st edn), Springer Nature, USA.
- ISTAT (2017) Diabetes in Italy. Years 2000-2016.
- Mohammed L, Jha G, Malasevskaia I, Goud HK, Hassan A, et al. (2021) The interplay between sugar and yeast infections: do diabetics have a greater predisposition to develop oral and vulvovaginal candidiasis? Cureus 13(2): e13407.
- Man A, Ciurea CN, Pasaroiu D, Ana-Ioana S, Felicia T, et al. (2017) New perspectives on the nutritional factors influencing growth rate of Candida albicans in diabetics. An in vitro study. Mem Inst Oswaldo Cruz 112(9): 587-592.
- Ende VM, Wijnants S, Dijck VP (2019) Sugar sensing and signaling in candida albicans and candida glabrata. Front Microbiol 10: 99.
- Graziottin A, Murina F, Gambini D, Stefania T, Barbara G, et al. (2020) Vulvar pain: The revealing scenario of leading comorbidities in 1183 cases. Eur J Obstet Gynecol Reprod Biol 252: 50-55.
- Bernstein JA, Seidu L (2015) Chronic Vulvovaginal candida hypersensitivity: An underrecognized and undertreated disorder by allergists. Allergy Rhinol 6(1): ar.2015.6.0113.
- Graziottin A, Gambini D (2017) Evaluation of genito-pelvic pain penetration disorder. In: WIW (ed.), The Textbook of Sexual Medicine, Springer Verlag, USA
- Farmer MA, Taylor AM, Bailey AL, Tuttle AH, MacIntyre LC, et al. (2011) Repeated Vulvovaginal Fungal Infections Cause Persistent Pain in a Mouse Model of Vulvodynia. Sci Transl Med 3(101): 101ra91-101ra91.
- Foster DC, Falsetta ML, Woeller CF, Pollock SJ, Kunchang S, et al. (2015) Site-specific mesenchymal control of inflammatory pain to yeast challenge in vulvodynia-afflicted and pain-free women. Pain 156(3): 386-396.
- Babula O, Linhares IM, Bongiovanni AM, Ledger WJ, Witkin SS, et al. (2008) Association between primary vulvar vestibulitis syndrome, defective induction of tumor necrosis factor-alpha, and carriage of the mannose-binding lectin codon 54 gene polymorphism. Am J Obstet Gynecol 198(1): 101.e1-4.
- Naglik JR, Gaffen SL, Hube B (2019) Candidalysin: discovery and function in Candida albicans infections. Curr Opin Microbiol 52: 100.
- Naglik JR, König A, Hube B, Gaffen SL (2017) Candida albicans- epithelial interactions and induction of mucosal innate immunity. Curr Opin Microbiol 40: 104-112.
- Kasper L, König A, Koenig P-A, GresnigtMS, Westman J, et al. (2018) The fungal peptide toxin Candidalysin activates the NLRP3 inflammasome and causes cytolysis in mononuclear phagocytes. Nat Commun 9(1): 4260.
- Jeziorek M, Frej-Mądrzak M, Choroszy-Król I (2019) The influence of diet on gastrointestinal Candida spp. colonization and the susceptibility of Candida spp. to antifungal drugs. Rocz Panstw Zakl Hig 70(2): 195-200.
- Graziottin A (2014) Recurrent cystitis after intercourse: why the gynaecologist has a say. In: Studd J, Seang LT, Chervenak FA (Eds.), Current Progress in Obstetrics and Gynaecology 2: 319-336.






























