Delayed Lactogenesis II of Unsuspected Origin
Luis Ángel Bolio-Molina1* and Gabriela Toledo-Verónico2
1Pediatrician, in private practice and in Cuernavaca General Hospital, Mexico
2Diplomate in Applied Practice Pediatrics, In private practice Cuernavaca General Hospital, Mexico
Submission: September 10, 2021; Published: September 21, 2021
*Corresponding author: Luis Ángel Bolio-Molina, Pediatrician, in private practice and in Cuernavaca General Hospital, Mexico
How to cite this article: Bolio-Molina LÁ, Toledo-Verónico G. Delayed Lactogenesis II of Unsuspected Origin. J Gynecol Women’s Health 2021: 22(2): 556082. DOI: 10.19080/JGWH.2021.22.556082
Summary
Introduction Delayed Lactogenesis II is the delayed and reduced milk production in the first ten days of exclusive breastfeeding that leads to, increasingly frequent, neonatal dehydration due to insufficient colostrum intake, as a consequence of a “bad breastfeeding technique”. However, in the peripartum, we observe a “negative water balance” that causes severe hypovolemia which, in turn, causes mammary hypoperfusion, leading to a postpartum milk shortage.
Objective: Establishing, clinically, that negative water balance causes hypovolemia and mammary hypoperfusion, leading to the late onset of Lactogenesis II.
Methods: Descriptive, observational, longitudinal, double-blind, and comparative study between 300 women, 150 postpartum, and 150 post-cesarean. We search prospectively and randomly, the indication of control of the water balance and its execution during the peripartum, in a second-level hospital in Cuernavaca, Morelos, Mexico, for 6 consecutive months.
Results: We found no indication or execution of water balance control, in 100% of both groups, its calculation was negative (3,38 L) and milk production was lower than expected (44.6 VS 100 ml/day), regardless of age, parity, mode of birth, socioeconomic status, occupation, schooling and desire to breastfeed.
Conclusions: We find that, in our environment, the control of the water balance in the peripartum is unusual, being negative in 100% of women with low milk production, which supports our hypothesis. Avoiding this negative water balance, we avoid mammary hypoperfusion, milk shortage, insufficient intake, and neonatal dehydration.
Keywords: Exclusive breastfeeding; Delayed Lactogenesis II; Insufficient milk syndrome; Neonatal hypernatraemic dehydration; Peripartum water deficit
Abbreviations: ND= Neonatal Dehydration; DLII= Delayed Lactogenesis II;IMS= Insufficient Milk Syndrome; PPH= Postpartum Hemorrhage; VC= Vascular Collapse; NWB= Negative Water Balance; GI= Group I; GII= Group II; WBC= Water Balance Control; VCP= Volume of Colostrum Produced; SSD p<0.05= Significant Statistical Difference with p<0.05; WB= Water Balance; IWD= Inadvertent Water Deficit; NDC= Neonatal Dehydration Criteria; EB= Exclusive Breastfeeding; ESLB= Exclusive “Successful and Lasting” Breastfeeding; TMB= Transient Mixed Breastfeedin
Introduction
Breastfeeding undoubtedly greatly benefits the mother-child binomial or dyad [1-4]. However, in our environment, in the last 20 years, the frequency of Neonatal Dehydration (ND) due to insufficient intake has increased, secondary to Delayed Lactogenesis II (DLII), both make up the Insufficient Milk Syndrome (IMS) [5,6]. The known causes of DLII are 1. “Bad breastfeeding technique”, the most accepted [5-7]; 2. Hypoprolactinemia, due to gestational pituitary hypoperfusion; 3. Retention of placental remains; 4. Postpartum hemorrhage (PPH) and; 5. Vascular collapse (VC) [8,9]. PPH and VC cause hypovolemia and pituitary hypoperfusion manifested as selective hypopituitarism and panhypopituitarism or Sheehan syndrome, respectively [7-11], which are underdiagnosed, despite the fact that both cause low or no milk production [12,13], which leads to ND for insufficient or no intake, in the first 10 days of life [14-17]. In the peripartum, we observed a Negative Water Balance (NWB), even larger than PPH and VC, which cause hypoperfusion and ischemia to non-vital organs, including the pituitary gland and mammary gland, by systemic circulatory redistribution, giving mammary hypoperfusion and, low or no milk production. Mammary blood flow doubles during pregnancy and lactation, positively related to milk production [17-19], if there are no adverse factors. Those who claim that low breast blood flow does not affect milk production, measured it in 55 women at 6 weeks of lactation, not at the beginning, and measured milk production, with the preprandial and postprandial weight of their babies. However, they contradictorily mention that breasts with little or no production have hypoflux [19], or hypoperfusion. We aim to establish and demonstrate, clinically, that there is a strong positive relationship between NWB and low milk production.
Material and Method
Our descriptive, observational, comparative and double-blind study consisted of longitudinally and prospectively reviewing the medical indications and nursing sheets, paired and randomly, in a non-probabilistic sample and for convenience of 150 postpartum women Group I (GI) and 150 post-cesarean women Group II (GII), in the Recovery and Gynecology areas of a second-level hospital in Cuernavaca, Morelos, Mexico, for 6 months, between September 2015 and February 2016, in the first three days of peripartum. Specifically, we seek the indication and execution of the Water Balance Control (WBC) of these women, as a means to know their blood volume and perfusion, since the NWB causes hypovolemia and mammary hypoperfusion.
We measured the Volume of Colostrum Produced (VCP), selfextracted manually, after explaining the technique and verbal consent, as a means of knowing the VCP that babies ingest, since NWB causes scarce VCP that results in insufficient intake leading to ND. Inclusion Criteria: a) Complete and legible medical indications of fasting, type, and volume of parenteral fluids supplied in the 72 hrs of peripartum; b) Indication and execution of WBC; c) Women without PPH and VC; d) Healthy women, without diabetes, hypertension or infection; d) No problems or breast surgery; e) With a healthy baby, without malformations.
Exclusion criteria: a) Incomplete and illegible indications; b) Diabetic, hypertensive, and infected women; c) With problems or breast surgery; d) With PPH (>0.5 L) and VC (>1.5 L) (8.9); e) With a sick, absent or malformed baby. Dependent variables: a) Fasting hours; b) Volume of liquids supplied; c) WBC (including PPH and VC); d) VCP; e) Patients’ weight on admission and discharge. Independent variables: a) Age, b) Parity, c) Schooling, d) Socio-economic level, e) Marital status, f) Occupation, g) Desire to breastfed.
Double-blind: 1. Randomly recording of indications and variables, ignoring the mode of birth; 2. Randomly collecting colostrum, not knowing variables and mode of birth. Once collected the information, we interrogated mode of birth to assign it to the corresponding group, until completing 150 per group. For the analysis, we use descriptive statistics, 2x2 and X2 tables. To compare by groups and independent samples, we used Student’s t, maintaining the statistical significance of 95%, with p<0.05. The data was handled with strict rules of confidentiality and ethics.
Results
During the selection, we excluded 46 participants until the sample was completed (n=300).
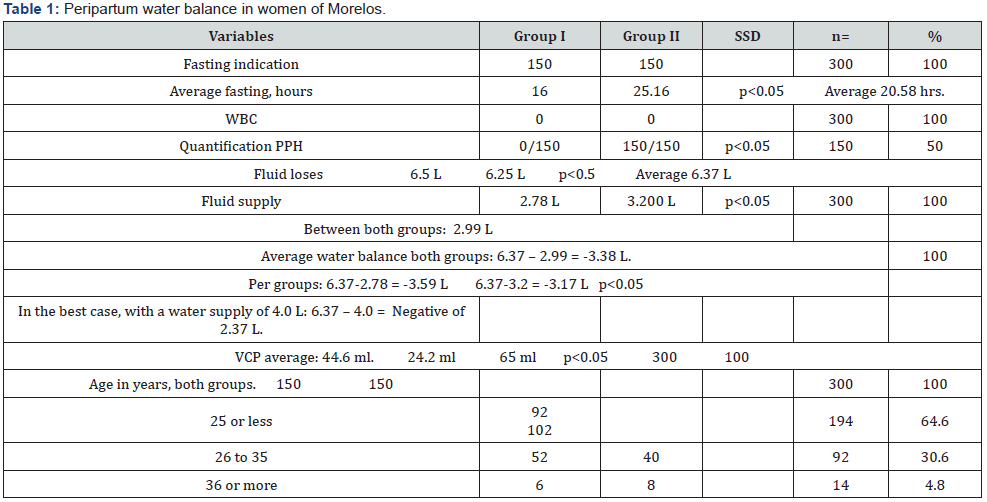
Indication and execution of WBC, absent in 100% of both groups.
Weight at admission, GI 40%, GII 80%, with Significant Statistical Difference with p<0.05 (SSD p<0.05). Without weight at discharge, 100% of both groups.
Average weight gain, subtracting the pre-pregnancy weight to the admission weight. GI 13.05kg (8.5 to 17.6), GII 12.5kg (6.3 to 18.7) (SSD p<0.05). Average both groups 12.77kg.
Water losses, GI 6.5kg, GII 6.25 (No SSDp<0.5). Average both groups 6.37kg.
Average fasting, GI 16hrs and GII 25.16 hrs (SSD p<0.05), with a range from 3 to 48hrs between both groups, an average of 20.58hrs.
Average fluid supplied volume 2.99 Liters (L) between both groups, ranges from 1 to 4L in 24hrs (L/d). Most commonly used liquids, in both groups, 5% glucose alone and alternated every 8 hours with Hartman, 1L of each.
PPH quantification absent in GI. In GII 100% during anesthesia only (p <0.05), average 500ml +/- 150 ml. None had CV.
We calculate the Water Balance (WB) based on the average of fluid supplied, 2.99L, minus the average of “obligatory” water losses, 6.37kg. It was negative (-) in both groups, average -3.38 L/d, which we call “Inadvertent Water Deficit” (IWD). GI -3.51 L, GII -3.26 L, with SSD p<0.05.
We collect colostrum, at least in two, of three feedings a day and, at least in 2, of 3 days of stay, in disposable syringes of 20ml, generic brand, measured the VCP and immediately supplied to the neonate.
The average VCP, between both groups, was 44.6ml in 24hrs (ml/d). Averages, GI 24.5 ml, GII 65 ml. Greater and significant range in multiparous with SSD p<0.05, but less than expected (100 ml/d) with SSD p<0.05.
Parity between both groups was 41.3% primiparous and 58.7% multiparous with SSD p<0.05.
By income, education, and occupation, 84% were low level, 16% lower-middle level and 0% of high level.
By age, between both groups, 64.4% were under 25 years old, with SSD p<0.05.
Desire of breastfeed, 100% of both groups (Table 1).
On admission, blood biometry was performed at 65% of GI and at 100% of GII (p<0.05). Prior to discharge at 5% of the GI and 15% of the GII, for suspicion of anemia.
100% of the neonates (n = 300) had neonatal dehydration criteria (NDC), minor criteria (under-hydration). None had major criteria (dehydration) since these appear from day 5 of life with exclusive breastfeeding (EB). Similar to our previous report [14].
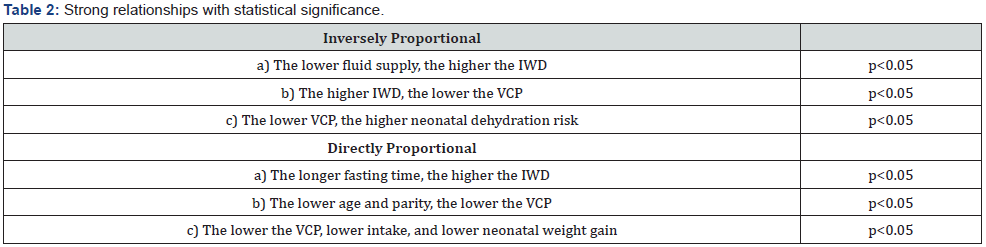
We found two types of strong relationships with SSD p<0.05 between both groups. 1. Directly proportional and 2. Indirectly proportional, as shown in Table 2.
Discussion
Pregnant women gain from 10 to 16.7kg of weight [20]. Prepregnancy gain and post-pregnancy loss are based on averages of 1. Newborn’s weight (3kg); 2. Placenta’s weight (500g); 3. Amniotic fluid volume (800-1,200ml); 4. Uterus’ weight (500- 900g); 5. Maternal fat deposits; 6. Extracellular fluid and blood volume (20). To the losses, we add 7. Loquios (200-500ml); 8. Loss of edema due to preeclampsia; 9. Prolonged fasting; 10. PPH>0.5L; 11. VC>1.5L and; 12. IWD= -3.38L.
The average weight gain is 13.35kg, 250-400g/week, from week 10 to 37 of gestation [21]. We assume that weight loss is equitable between hydric and non-hydric losses (50/50), 6.675 L, and kg, respectively. The daily fluid supply recommended for pregnant women is 4.8L/d, and for lactating women 3.3L/d [22,23]. We found an average supply of 2.99L/d for both groups, between 1 and 4L/d. GI 2.78 L/d, GII 3.2 L/d, significantly lower than recommended, with SSD p<0.05.
Comparing the average of water losses found, 6.37L, with the average of liquids supplied, 2.99L/d, we have an NWB of 3.38L (6.37-2.99), statistically higher than PPH and VC. If we would provide the recommended volume for pregnant women, 4.8L/d, and contrast it with the average of fluids supplied, 2.99L/d, we would also have an NWB of 1.81L (4.8-2.99), with SSD p<0.05. Contrasting water losses and recommended volume with the NWB averages by groups, we have GI 6.37-2.78=-3.59L, and 4.8- 2.78=-2.02L; GII 6.37-3.2=-3.17L, and 4.8-3.2=-1.6L, both with SSD p<0.05.
In the best of cases, contrasting them with the maximum fluid supply found of 4 L, there are still NWB, 6.37-4=-2.37 L, and 4.8- 4=-0.8 L, with SSD p <0.05. Without WBC, it will be difficult to know how many women had PPH or VC, both determining factors of hypovolemia and systemic circulatory redistribution [7-9]. Discarding both, the IWD that we found, it is significantly higher than the PPH and the VC, therefore, it causes hypovolemia and hypoperfusion, or mammary hypo-flow, conditioning scarce VCP, whose average was 44.6ml/d, similar to our previous reports, but less than the expected minimum (100ml/d) the first 5 days of EB [12-14].
In the last 20 years, we have observed a lack of WBC in women in peripartum, which “masks” an IWD that implies mammary hypoperfusion, Delaying Lactogenesis II, which depends directly on irrigation, which supplies substrates and water, of the plasma, to each alveolar cell, for synthesize all the components of breast milk [17-19,24,25].
Hypoprolactinemia is mainly secondary to pituitary hypoperfusion, rather than poor or infrequent sucking of the maternal nipples by the newborn. To date, there is no convincing evidence that increasing suction frequency improves milk production, in most cases, during the first 10 days of EB, only the level of prolactin increases [5-7] but not milk production, so prolactin only plays a permissive role [17].
Supported in the literature, in our observations, and in our analysis, we consider that “failure in breastfeeding is the clinical manifestation of DLII” [12-14], and that this, in turn, “is secondary to mammary hypoperfusion by IWD”, more than to other factors, at least in the first 10 days of lactation and postpartum. In other words, we dare to say that “the VCP is directly proportional to the mammary perfusion”, that is, “the greater the mammary perfusion, greater is the VCP, and vice versa”. Mammary hypoperfusion due to hypovolemia or IWD, in the peripartum, is due to a lack of WBC. We can say that “the failures in milk production originate by an NWB”, or maternal dehydration [17].
Running women’s WBC in the peripartum avoids IWD and with this, we avoid “NWB, mammary hypoperfusion and DLII”. We believe that “the milk coming in” occurs at the end of the second week of the puerperium because, during this period, women recover their water balance by normalizing their diet, which, in turn, normalizes the circulatory volume and, consequently, mammary perfusion. If we add to this the greater strength, quality, and frequency of sucking of the newborn, we have the increase in milk production [12-14,17], “as it normally happens”. Until then, an Exclusive, “Successful and Lasting” Breastfeeding (ESLB) will be possible.
In our setting, it is unlikely that empirical midwives and health personnel from maternity wards publics, and privates, take care of the WBC of women in peripartum. Even in second-level hospitals, they do not quantify PPH or take care of WBC. We lack the “culture of prevention of hypovolemia or IWD” because we are unaware of its risks and consequences.
Historically and empirically, in rural and suburban areas of Mexico, postpartum women are provided with “liquids” in the form of teas, “atoles”, chicken broth, and even pulque and beer, for the “belief” that they are galactagogues. In fact, they add volume, at least part of the IWD. Simultaneously, newborns are provided with tea, corn atole, cow’s milk, or formula, according to the family economy, while “the milk coming in” occurs [12-14]. With these practices, both are being partially hydrated, avoiding DLII and ND, or IMS [14-16]. By promoting EB and rooming-in, as the WHO indicates, these customs are disappearing, which contributes to the increase of binomials with IMS, which implies more hospital admissions of dehydrated and anemic mothers, and of dehydrated, icteric and feverish neonates, classified as “Septics” [12]. We must recognize that these ancient empirical practices, historically, saved the lives not of thousands, but of millions of people who today are nurses, doctors, pediatricians, and other professionals, born before the implementation of the Baby-Friendly Hospital Initiative in 1991. The “cribs rooms” also disappeared, where the neonates were fed with 30ml of formula every 3 hours and there were few cases of ND. We must remember that Lactogenesis II begins 36 to 72hrs after the placenta is expelled, and is delayed when retained remains [6-11,17,18]. During this time, the lactogen and other placental hormones decrease and stop inhibiting the secretion of prolactin and its stimulating effect on the breast to initiate milk production [7-9,17]. We believe that, while this occurs, there is “physiological hypoprolactinemia”, which becomes pathological when the woman suffers from hypovolemia due to IWD, PPH, VC or retention of placental remains. Prolactin and VCP do not increase only with early and frequent sucking of the newborn, from the first half-hour of life. Both will increase as placental inhibitors progressively decrease and maternal hydration and mammary perfusion improve. By avoiding IWD, we will avoid in the short term mammary hypoperfusion and DLII.
Let us also remember that newborns experience a highly stressful “transition or survival process”. 1. The first period of reactivity, the first 30 minutes of life; 2. Period of sleep and quiet, the next 2 to 6 hours and; 3. Second reactivity period, after 6 hours [26], of which they recover in 24 hours [17]. Based on this, and from our particular perspective, it is not natural that newborns should start sucking, from the first half-hour of life, being in their stressful process of survival, and subsequent adaptation to extrauterine life, during which they are not hungry because they maintain their fetal reserve. They can suck, but unproductively, due to DLII secondary to 1. Placenta expelled recently or ongoing; 2. High placental inhibitors; 3. Low prolactin; 4. Pituitary hypoperfusion; 5. Mammary hypoperfusion; 6. Prolonged fasting; 7. Insufficient water supply; 8. IWD; and perhaps, 9. PPH; 10. VC; 11. Retention of placental remains.
We are convinced that if a healthy term newborn starts sucking in its first half-hour of life, during its stressful, transition or survival process, it will also suffer despair and fatigue, because of what we call “vacuum suction” which increases their requirement hydric and caloric, another cause of ND [12-14]. Recently unwanted consequences of steps 4, 6, 7, and 9 from the originals “Ten Steps to Successful Breastfeeding” are mentioned because they lack reproducible scientific support [27,28]. For us, IMS manifested by low milk production, low intake [12], jaundice [13], and dehydration is a consequence of omitting or ignoring step 6, in neonates with NDC [14], which are unknown by the health personnel, whose consider them normal.
On the other hand, women, in addition to the pain of childbirth or cesarean section, suffer despair, fatigue, and depression [7,17,29]. We add “frustration” when they feel cannot successfully breastfeed their babies. Even “guilt” for not meeting that expectation, especially if, instead of “Comprehension”, they feel “Pressure” from breastfeeding support groups, and of their families, which makes them feel “bad mothers” because we often forget her feelings. Maternal stress has been shown to cause vasoconstriction [7,29], which is added to that caused by hypovolemia, resulting in mammary hypoperfusion and DLII.
“The milk coming in” occurs “physiologically” at the end of the second week of EB [12-14,17]. It has recently been mentioned that lower efficiency and greater variation in the duration of nighttime sleep [30], as well as alcohol consumption and postpartum maternal depression [31], can also cause DLII. There is no convincing evidence of better results to accelerate and increase milk production, increasing the sucking frequency of the newborn, or using galactagogue medications [32]. Even mothers of premature infants of 30 weeks gestational age, treated with Domperidone for 14 days, from day 8 postpartum, only increase their milk production by 50% [33], the same occurs without this treatment.
Based on the literature, on our observations, on the analysis we carry out, and until proven otherwise, as a timely solution to the DLII, we carry out and suggest the following: 1. Avoid NWB or IWD in the peripartum and 2. Allow Transient Mixed Breastfeeding (TMB) [12-14] with syringe, spoon, dropper or, preferably, with a “feeding tube” rather bottle, supplying the newborn with milk extracted from his mother or from the bank, or formula, at least in the first ten days of lactation, the time necessary for the normalization of maternal water balance, thereby improving mammary perfusion, resulting in increased VCP, and promoting greater chances of establishing an ESLB. We believe that “wellhydrated mothers have a higher colostrum production and a lower risk of dehydrated neonates”.
Aware of the limitations of our clinical, descriptive, and “single-center” study, but also of its strengths such as prospective, randomized, comparative, and double-blind, in addition to being easy reproduction, and 100% demonstrable we propose it as the basis for future multicenter studies with a larger sample.
Conclusion
In our environment, Water Balance Control is not indicated nor executed in the peripartum. It is negative in women with low milk production, due to hypovolemia secondary to 1. IWD due to prolonged fasting; 2. Insufficient liquids supply; 3. PPH and; 4. VC. All produce mammary hypoperfusion, which delays and reduces milk production in the first ten days of EB. Consequently, we have more hospital admissions of dehydrated, and anemic mothers, and of dehydrated neonates, many with catastrophic hypernatremia. We suggest two prophylactic actions, immediate and free, that would greatly reduce maternal and neonatal morbidity and mortality in the peripartum: 1. Make WBC mandatory, to avoid IWD and, 2. Provide TMB during the first ten days, when we suspect IMS.
Acknowledgment
To our son Uriel, for having been a “guinea pig” in several of our research works. Thank God for allowing us to love and enjoy what we do and for allowing us to share it for the benefit of children.
Conflicts of Interest
a) The authors declare that they have no conflicts of interest in carrying out and disseminating this research work.
b) Presented at the Advanced Pediatric Research Contest, poster modality, at the 50th National Congress of Pediatrics of Mexico (CONAPEME), Acapulco, Guerrero. Mexico. From May 3 to 6, 2018.
c) First place in Pediatric Research, in the Free Work Contest, Poster mode, during the XXII Regional Congress of the Pediatric Federation of the Center (FEPECE) and V International Congress of the College of Pediatrics of the state of Mexico, Toluca, State of Mexico, September 2018.
Oral presentation at 2nd. International Congress on Health Sciences Research and 5th International Congress on Nursing Research Topic. Taught by the School of Higher Studies of Tetecala of the Autonomous University of the State of Morelos, August 29 and 30, 2019. Tetecala, Morelos. Mexico.
References
- Wouk K, Lara-Cinisomo S, Stuebe AM, Charles P, Petrick JL, et al (2016) Clinical interventions to promote breastfeeding by latinas: A Meta-analysis. Pediatrics137(1): e20152423.
- Alzaheb RA (2016) Factors associated with the initiation of breastfeeding within the first 48 hours of life in Tabuk, Saudi Arabia. Int Breastfeed J 11: 1-6.
- Bustos-Lozano G, Flores-Antón B (2016) Aspectos prácticos de la extracción, conservación y administración de la leche materna en el hogar. Act Pediatr Esp 74(7): e149-e158.
- Nguyen T, Dennison BA , Fan W, Changning X, Birkhead GS, et al (2017) Varition in formula supplementation of breastfed newborn infants in New York Hospitals. Pediatrics 140(1): e20170142.
- Sultana A, Rahaman KU, Manjula S MS (2013) Clinical update and treatment of lactation insufficiency. Med J Islamic World Acad Sci 21(1): 19-28.
- Livingstone V (1996) Post-Partum breastfeeding assessment. J SOGC 18: 141-153.
- González GLG, Carrera GL, Arias LRP, Costa RM, Suárez RM, et al. (2016) Deshidratación hipernatrémica asociada a la alimentación con lactancia materna en el periodo neonatal. Acta Pediatr Esp 74(10): 261-265.
- Willis CE, Livingstone V (1995) Infant insufficient milk syndrome associated whit maternal postpartum hemorrhage.
J Hum Lact 11(2): 1-5. - Thompson JF, Heal LJ, Roberts ChL, Ellwood DA (2010) Women's breastfeeding experiences following a significant primary postpartum hemorrhage: A multicenter cohort study. Int Breastfeed J 5(5): 1-12.
- Contreras-Zúñiga E, Mosquera-Tapia X, Domínguez-Villegas MC, Parra-Zúñiga E (2009) Síndrome de Sheehan: descripción de un caso clínico y revisión de la literatura. Rev Colomb Obstet Ginecol 60(4): 377-381.
- Anfuso S, Patrelli TS, Soncini E, Chiodera P, Fadda GM, et al. (2009) A case report of Sheehan’s syndrome with acute onset, hyponatremia and severe anemia. Acta Biomed 80: 73-76.
- Bolio MLA (2013) Lactogénesis en los primeros cinco días del puerperio y la lactancia. Rev Mex Pediatr 80(1): 10-14.
- Bolio-Molina LÁ (2016) Ictericia en neonatos sanos con lactancia materna exclusiva por madres con baja producción lá Vox Paediatrica 13(1): 29-33.
- Bolio-Molina LÁ (2017) Criterios de deshidratación neonatal secundaria a lactancia materna exclusiva. Vox Paediatrica 24(1): 13-18.
- Bischoff et al (2017) Treatment of hypernatremia in breastfeeding neonates: A Systematic Review. Biomed Hub 2(1): 454980.
- Mujawar NS, Jaiswal AN (2017) Hypernatremia in the newborn: neonatal hypernatremia and hypernatremic dehydration in newborns who receive exclusive breastfeeding. Indian J Crit Care Med 21(1): 30-33.
- Hajela R (2015) Understand lactation and lactation Failure: Fight the curse of insufficient breast milk J App Med Sci 3(9B): 3289-3301.
- Holanda AAR, Gonçalves AKS, Medeiros RD, Oliveira AMG, Maranhão TMO, et al. (2016) Ultrasound findings of the physiological changes and most common breast diseases during pregnancy and lactation. Radiol Bras 49(6): 389-396.
- Geddes DT, Aljazaf KM, Kent JC, Prime DK, Spatz DL, et al (2012) Blood flow characteristics of the human lactating breast. J Hum Lact 28(2): 145-152.
- Herring SJ, Oken E (2010) Ganancia de peso durante el embarazo: Su importancia para el estado de salud materno-infantil. Ann Nestlé 68: 17-28.
- Almira GA (2010) Variación del peso materno en el embarazo. Artículo de revisió Medisan 14(1): 71.
- Figueróa-Damián R, Beltrán-Montoya J, Espino y Sosa S, Reyes E, Segura-Cervantes E, et al. (2015) Consumo de agua en el embarazo y la lactancia. Acta Pediatr Mex 34: 102-108.
- (2005) Water, Sanitation and health protection and the human environment World Health Organization Geneva.
- https://www.unicef.cl/lactancia/.../FISIOLOGIA%20DE%20LA%20GLANDULA
- Aguilar-Palafox MI, Fernández-Ortega MA, Lactancia materna exclusiva. Monografí Facultad de Medicina de la UNAM.
- Montiel-Morales DP, Ferreira-Jaime F, Rendón-Macías ME (2016) Comparación del periodo de transición en recién nacidos obtenidos de parto en agua y parto seco. Estudio de cohortes. Rev Mex Pediatr 83(5): 148-153.
- Moreno VJM, Galiano SMJ (2016) Consecuencias no deseadas de las iniciativas actuales de lactancia materna. Bibliografía comentada. Acta Pediat Esp 74(9): 240-241.
- Bass JL, Gartley T, Kleiman R (2016) Unintended consequences of current breastfeeding initiatives. JAMA Pediatrics 170(10): 923-924.
- Fallon V, Groves R, Halford JCG, Bennet KM, Harrold JA, et al. (2016) Postpartum anxiety and infant-feeding Outcomes: A systematic review. J Hum Lact 32(4): 740-758.
- Casey T, Sun H, Burgess HJ, Crodian J, Dowden S, et al. (2019) Delayed lactogenesis ii is associated with lower sleep efficiency and greater variation in nightly sleep duration in the third trimester. J Hum Lact 35(4): 713-724.
- Oliveira-Rocha B, Penido-Machado M, Lima-Bastos L, Santos AP, Santos LC, et al. (2020) Risk factors for delayed onset of lactogenesis ii among primiparous mothers from a brazilian baby-friendly hospital. J Hum Lact 36(1): 146-156.
- The Academy of Breastfeeding Medicine (2011) ABM Protocolo Clínico # 9: Uso de Galactogogos para Iniciar o Aumentar la Tasa de Secreción de Leche Materna.
- Asztalos EV, Kiss A, daSilva OP, Campbell-Yeo M, Shinya I, et al. (2019) Role of days post delivery on breast milk production: a secondary analysis from the EMPOWER trial. Int Breastfeed J 14(21): 1-7.






























