Ovulation Induction in Patients with Polycystic Ovarian Syndrome (PCOS) & Hyperprolactinemia (HPRL): Efficacy of Letrozole (LE) Combined with Cabergoline (CE) in Comparison to (LE) Alone
Aisha Mohamed Elbareg¹* and Fathi Mohamed Essadi²
1Associate Clinical Professor & Senior Consultant, Department of Obstetrics and Gynecology, Al-Amal Hospital / Faculty of Medicine, Misrata University, Libya
2Senior Consultant Obstetrician & Gynecologist, Head of Department, Misrata Medical Centre, Libya
Submission: May 05, 2021; Published: May 11, 2021
*Corresponding author: Aisha Mohamed Elbareg, Associate Clinical Professor & Senior Consultant, Department of Obstetrics and Gynecology, Al-Amal Hospital / Faculty of Medicine, Misrata University, Libya
How to cite this article: Aisha M E, Fathi M E. Ovulation Induction in Patients with Polycystic Ovarian Syndrome (PCOS) & Hyperprolactinemia (HPRL): Efficacy of Letrozole (LE) Combined with Cabergoline (CE) in Comparison to (LE) Alone. J Gynecol Women’s Health. 2021: 21(4): 556066. DOI: 10.19080/JGWH.2021.21.556066
Abstract
Objectives: (PCOS) is the most common cause of anovulatory infertility, with majority of patients having mild (HPRL). (CE), a dopamine receptor agonist, inhibits prolactin secretion, leading to better ovulatory response. (LE), an aromatase inhibitor, without adverse effects on endometrium & induces fewer mature follicles with less risk of OHSS. Our aim was to investigate effects of combined (LE) & (CE) in comparison to (LE) alone on ovulation & clinical pregnancy rates in (PCOS) patients with (HPRL).
Patients & Methods: 180 women with (PCOS) and of 22-38 years old, were enrolled in a hospital based clinical trial. Patients randomly allocated into 2 groups, (A&B). All with a serum prolactin > 32 ng/ml. Patients in (A): (92) were given (LE), 5mg for 5days: (3 - 7 of the cycle)/3 cycles and (CE), 0.5mg weekly for 12 weeks. Those in (B): (88) received only (LE), same dose & duration as in (A). All patients were matched for their age and BMI. Exclusion criteria: other causes of (HPRL). Outcome measure: ovulation rate & detection of both chemical & clinical pregnancies by βhCG and ultrasound of fetal cardiac activity, 2-4 weeks after missed period. Follow-up for 6 months. Data analysis by using SPSS version for windows, P-value significant if (< 0.05).
Results: 3 patients from (A) & 5 from (B) had drug side effects and were excluded. None of patients were lost during the follow-up period. In (A), difference between mean serum prolactin level before & after treatment was statistically significant (P<0.001): 48.3±4.2ng/ml and 8.1±5.2ng/ml, respectively. No significant decrease of prolactin level in (B) (P >0.05). After treatment, BMI in (A) 24.1± 3.2, & 24.2 ± 3.6 in (B) (P=0.567). (56.2%) of women in (A) became regularly menstruating but only (30.1%) in (B) (P< 0.05). Ovulation rate was higher in (A) (50.6%) in comparison to (B) (26.5%), (P<0.05). Clinical pregnancy rate in (A) (41.6%) and (21.6%) in (B) (P<0.05). Neither twin pregnancy, nor OHSS were recorded in both groups.
Keywords: Polycystic Ovarian Syndrome; Hyperprolactinemia; Ovulation Induction; Letrozole; Cabergoline
Abbreviations: PCOS: Polycystic Ovarian Syndrome; HPRL: Hyperprolactinemia; LE: Letrozole; CE: Cabergoline
Introduction
(PCOS) and (HPRL) are the most common endocrine disorders in women of reproductive age. According to the World Health Organization (WHO) estimation revealed over 116 million women (3.4%) are affected by (PCOS) worldwide [1]. Owing to the intricacy of this condition, various sets of diagnostic criteria have been initiated for the confirmation of (PCOS) which are: National Institute of Health (NIH,1990), Rotterdam’s Criteria (2003), Androgen Excess and PCOS society (2006), NIH 2012 extension of ESHRE/ASRM 2003(2012) [2]. The prevalence of (PCOS) is estimated at about 4 to 21 % when it is diagnosed according to the Rotterdam criteria. It accounts for 75% of cases with anovulatory infertility [3-5]. At least two of the following three features should be present for proper (PCOS) diagnosis: ovulatory dysfunction (oligomenorrhea and/or anovulation,); hyperandrogenemia (biochemical feature of androgen excess) or hyperandrogenism (the clinical feature of androgen excess); polycystic appearance of ovaries on ultrasonography together with the exclusion of other etiologies [6]. Menstrual irregularity approximately in all obese and 79% of thin (PCOS) patients [7]. Prevalence of (HPRL) is not rare in young women with menstruation-related problem; it varies according to age and manifestations. The most common hypothesis to explain the link between (HPRL) & (PCOS) is a possibly a common hypothalamic-pituitary abnormality for both [8]. It varies from 0.4% in the normal adults or 2.9% in women with adult-onset amenorrhea or up to 75% in women with both amenorrhea & galactorrhea [9].
The association between (HPRL) and (PCOS) has been described since 1950s and has suggested the existence of a pathophysiological link between these two entities but data from literature on this subject was unclear. 30% of patients with (PCOS) show a modest rise in prolactin level [10-13]. Increasing serum prolactin in these patients could be detected in both follicular and luteal phase of the normal and stimulated cycles. Dopamine release from the hypothalamus inhibits prolactin secretion, and it also affects the secretion of gonadotropins. When this inhibitory effect of dopamine is reduced, prolactin secretion will increase in addition to abnormalities in gonadotropins including luteinizing hormones (LH). (CE) which is a dopamine receptor agonist, with higher affinity to dopamine D2 receptors had improved uterine perfusion and achieved a better ovulatory response in (PCOS) patients [14], due to its ability to inhibit the vascular endothelial growth factor (VEGF) secretion in luteinized granulosa cells [15]. Other studies concluded that (CE) use could normalize androgen level and therefore improve the menstrual irregularity in women with (PCOS) [16].
(LE) is a third-generation aromatase inhibitor. It blocks the conversion of C-19 androgens to C-18 estrogens by competitively inhibiting the enzyme, aromatase (cytochrome P-450), which is an essential step in estrogen biosynthesis in the ovary and other tissues [17]. The subsequent feedback to the hypothalamus containing reduced estrogen levels, triggers a compensatory increase in hypothalamic gonadotropin-releasing hormone (GnRH) secretion, and thus an increased release of pituitary gonadotropins (FSH) & (LH.). These gonadotropins subsequently promote growth of the follicles and stimulate ovulation. (LE) has 99.9% bioavailability after oral administration. It has a single dose terminal half-life of 42h.
As the combined use of (CE) which normalizes prolactin level and (LE) which stimulates ovulation, could be effective in management of menstrual cycle irregularities, ovulation induction and increase pregnancy rate in (PCOS) patients, therefore, in this in this study, we will investigate their efficacy and the reproductive outcomes in those patients in comparison to (LE) alone.
Methods
This randomized clinical trial was conducted on 180 women with (PCOS) aged between 22 and 38 years, referred to the consultation clinics at Al Amal Hospital & Gynecology and Obstetrics department, Misrata Medical Centre, in the year of 2018/2019. All women showed increased serum prolactin >32ng/ ml. Ex criteria: other causes of increased prolactin levels such as hypothyroidism, pituitary tumors, patients who had history of cardiovascular disease and history of using prolactin increasing drugs. Study protocol was approved by the ethics committee of the hospital. All women were subjected to history taking and physical examination, with a written consent was taken from each case.
Patients were randomly allocated into two groups. Women in group (A) received (LE), 5mg daily for 5 days, from day 3 of the cycle up to 7th day/3 cycles, in addition to (CE), 0.5mg weekly for 12 weeks, those in group (B) received only (LE), same dose and duration as in (A). Serial ultrasound monitoring was conducted for detection of ovulation throughout the course of therapy starting from day 10 of menstrual cycle. Depending on follicular size (18-24mm) and endometrial thickness, human chorionic gonadotropin (hCG) was administered. The main outcome measures were the effect on BMI, rate of ovulation and detection of both biochemical & clinical pregnancy by estimation of (βhCG) and ultrasound detection of fetal cardiac activity, 2-4 weeks after missed period. Follow-up period was for six months. Statistical analysis of data performed using SPSS version for windows. P-value considered significant if (< 0.05).
Results
Three women from group (A) and five from group (B) had drug side effects and were excluded from the study. None of patients in either group were lost during the follow-up period. Data of (89) patients in Group (A) and (83) patients in group (B) were analyzed, including age, BMI, serum prolactin, and type of infertility. Regarding the age, the mean ±SD in group (A) was 24.4 ± 2.7 years, while in group (B), was 24.6 ± 2.8 years, (P=0.926). BMI: the mean ±SD was 29.8 ± 2.7 kg/m² in group (A), while in group (B), was 29.5 ± 2.6 kg/m² (P=0.885). Serum prolactin in group (A) was 48.3 ± 4.2 ng/ml compared with 48.2 ± 3.5 ng/ml in group (B) (P=0.721). All patients in both groups had irregular cycles (Table 1).
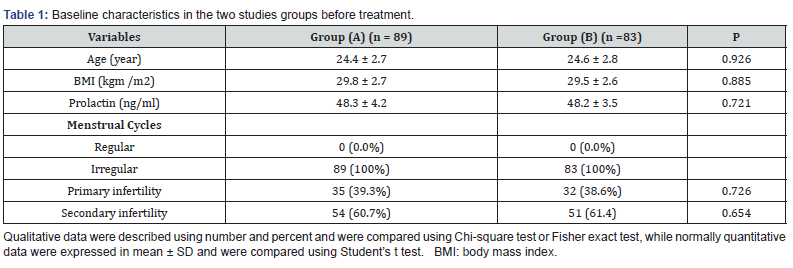
The women in group (A) received (LE), 5mg daily from day 3 to day 7 of the cycle for 3 cycles and (CE), 0.5 mg weekly for 12 weeks. Results revealed significant difference in BMI, the mean level of serum prolactin, regulation of the cycle, rate of ovulation & pregnancy. BMI significantly reduced from 29.3 ± 4.7 kg/m² before treatment to 21.1 ± 3.2 kg/m² after treatment (P<0.05). Serum prolactin level was significantly reduced from 48.3 ± 4.2 ng/ml before treatment to 8.1 ± 5.2 ng/ml after treatment (P<0.001). Also, before treatment all women had irregular cycles, whereas after treatment, 50 (56.2%) patients became regularly menstruating (P<0.001) (Table 2).
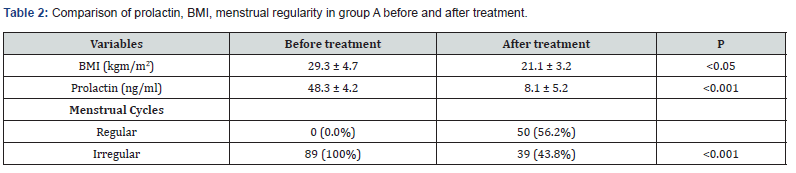
In group (B), women received only (LE), same dose & duration as in (A). Results showed that the mean BMI decreased significantly after treatment, {from 29.5±2.6 kg/m² to 23.1 ± 3.6 kg/m² (P<0.05)}. Before (LE) all women had irregular cycles but became regular after treatment in 25 (30.1%) patients (P<0.05). The mean of prolactin level did not show significant difference before & after treatment (P= 0.349.) (Table 3).
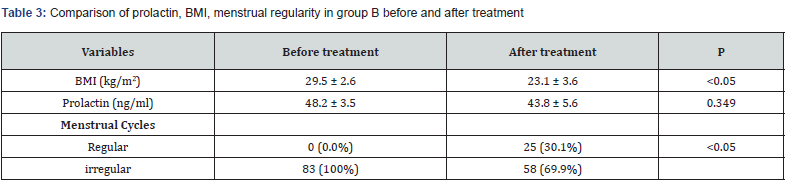
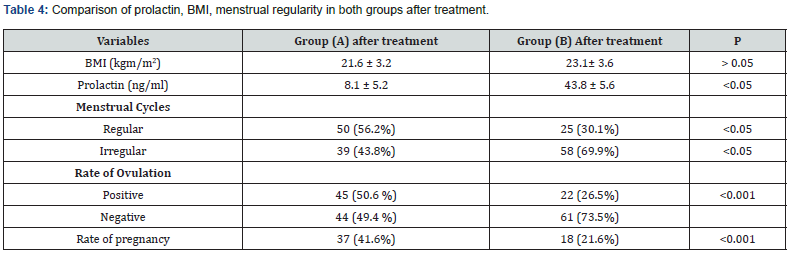
Comparison between the two studied groups after treatment showed no statistically significant difference between them in BMI, (21.6 ± 3.2 kg/m² in (A), and 23.1 ± 3.6 kg/m² in (B) (P > 0.05). The change in the mean level of prolactin was significantly different, in group (A) was 8.1 ± 5.2 ng/ml, while in (B) was 43.8 ± 5.6 (P<0.001). All patients in (A) & (B) had irregular menstrual cycles; there was significant difference after treatment, 50 (56.2%) of women in (A) became regularly menstruating in comparison to 25 (30.1%) patients in (B) (P< 0.05). Such observation raised the possibility of the (CE) use in cases of cycle irregularity. The rate of ovulation and pregnancy were also studied. Positive ovulation was seen in 45 (50.6%) women in group (A), compared with 22 (26.5%) in group (B) (P<0.001). Pregnancy was also detected either biochemically or clinically in 37 (41.6%) patients from (A), compared with only 18 (21.6%) in group (B) with (P <0.05) (Table 4) (Figure 1).

Discussion
To the best of our knowledge no study has been previously conducted to evaluate the effect of (LE) plus (CE) for induction of ovulation in infertile (PCOS) patients. Therefore, this study was carried out to evaluate the effect on the menstrual irregularity, and reproductive outcomes of adjunctive use of (CE) with (LE) in infertile (PCOS) patients with (HPRL) in comparison to (LE) alone. The ESHRE-ASRM 2018 consensus guideline has recommended that (LE) should be used as the first-line ovulation induction agent in the management of anovulatory infertility due to (PCOS) (rather than clomiphene) unless (LE) is not available [18]. It is an aromatase inhibitor with a dual inhibition of conversion of androstenedione to oestrone and testosterone to oestradiol, together with systemic prevention of oestrogen biosynthesis. It thus initiates feedback to the hypothalamic-pituitary axis with a resultant rise in gonadotropin production to induce ovulation [19], without having antiestrogenic effects on endometrium and less risk of multi follicular development. Furthermore, it has 99.9% bioavailability after oral administration. It has a single dose terminal half-life of 42h, but a steady-state concentration is not achieved for 2 to 6 weeks (steady-state half-life is 118h) [20], therefore, it is rapidly eliminated from the body leading to late follicular rise in circulating estrogen thereby enhancing development with subsequent increase in pregnancy. (CE) is a long- acting dopamine agonist and has higher affinity to dopamine D2 receptor with a serum half-life of 43-hour limit, so it is effective in the treatment of patients with (HPRL) [21,22]. However, few studies have been performed using (CE) in patients with (PCOS) [23-26].
As expected, our results showed that the mean serum level of prolactin decreased in the group treated with (CE) and the difference was significant after treatment as compared with the other group who did not receive it. It has been found that there was a significant relation between basal levels of prolactin and the changes in prolactin level due to inhibitory effect of bromocriptine and L-Dopa and suggested that the reduction in the inhibitory effect of dopamine in the hypothalamus can be a reason for inappropriate increased levels of LH and prolactin in (PCOS) patients with (HPRL) [27]. The results of our study showed higher rate of cycle regulation in patients with (PCOS) after treatment with (CE) which was consistent with those observed by Ghaneei et al who concluded that (CE) can be administered safely in (PCOS) patients with (HPRL) to improve the menstrual cycles [28]. Although the mechanism is not clear, one of the proposed mechanisms is that (CE) as a long-acting agonist of dopamine, which has high affinity to dopamine receptors D2, inhibits the vascular endothelial growth factor (VEGF) secretion in luteinized granulosa cells both in vitro and vivo [15]. Another possible mechanism is that treatment by (CE) restores ovarian function due to its inhibitory effect on LH seretion and androgen concentration [29]. Our results also showed increasing the rate of ovulation and therefore pregnancy rate as it has been found before because of dopamine agonist can increase perfusion in (PCOS) patients with (HPRL), decrease in their LH level, increase in ovulatory cycles, and finally regulate their menstrual cycles. Ajoss et al. [21] showed that using (CE) can increase the uterine perfusion in (PCOS) patients. The results of Paoletti and colleagues showed that the use of (CE) 0.5mg/week for 4 months in the treatment of (PCOS) patients can cause a decrease in the level of LH and improve irregular menstrual cycles, but the recovery rate was not mentioned [23]. These studies confirm our results, although a similar study which used the combination of (LE) and (CE) in (PCOS) patients was not found.
Conclusion
In conclusion, (PCOS) is one of the commonest endocrinopathies affecting females leading to anovulation and infertility and the use of (CE) & (LE) in induction of ovulation in those patients who also have (HPRL), results in high ovulation and pregnancy rates and is superior to (LE) alone, therefore, we suggest that the use of the combination of (LE) and (CE) in the management of PCOS patients is highly effective and safe in regulating the menstrual cycles, improving ovulation and pregnancy rates, and should be used as the first-line treatment for them.
Author Contributions
Clinical work conducted by AME & FME in addition to: a) AME- designed the study, data collection, statistical analyses/Interpretation, and writing – original draft Manuscript. FME- data collection, Literature Search, and critical review. All authors approved the final manuscript.
References
- Bharathi RV, Swetha S, Neerajaa J, Madhavica JV, Janani DM, et al. (2017) An epidemiological survey: Effect of predisposing factors for PCOS in Indian urban and rural population. Middle East Fertility Society Journal 22(4): 313-316.
- Bulsaraa J, Patel P, Soni A, Acharya S (2021) A review: Brief insight into Polycystic Ovarian syndrome. Endocrine and Metabolic Science 3: 1-7.
- Lizneva D, Suturina L, Walker W, Brakta S, Gavrilova-Jordan L, et al. (2016) Criteria, prevalence, and phenotypes of polycystic ovary syndrome. Fertil Steril 106(1): 6-15.
- Fritz MA, Speroff L (2011) Clinical gynecologic endocrinology and infertility. In: (8th edn), Wolters Kluwer Health/Lippincott Williams & Wilkins, Philadelphia, pp. 1296-1307.
- (2013) Practice Committee of the American Society for Reproductive Medicine. Use of clomiphene citrate in infertile women: a committee opinion. Fertil Steril 100(2): 341-348.
- Bachelot A (2016) Polycystic ovarian syndrome: clinical and biological diagnosis. Ann Biol Clin (Paris) 74(6): 661-667.
- Toosy S, Sodi R, Pappachan JM (2018) Lean polycystic ovary syndrome (PCOS): an evidence-based practical approach. Journal of Diabetes & Metabolic Disorders 17(2): 277-285.
- Delcour C, Robin G, Young J (2019) PCOS and Hyperprolactinemia: what do we know in 2019?
Clin Med Insights Reprod Health 13: 1-7. - Lee DY, Oh YK, Yoon BK, Choi DS (2012) Prevalence of hyperprolactinemia in adolescents and young women with menstruation related problems. Am J Obstet Gynecol 206(3): 213. e1-5.
- Duignam NM (1976) Polycystic ovarian disease. Br J Obstet Gynaecol 83(8): 593-602.
- Falaschi P, Frajese G, Rocco A, Toscano V, Sciarra F, et al. (1977) Polycystic ovary syndrome and hyperprolactinemia. J Steroid Biochem 8: 12.
- Coremblum B, Taylor PJ (1982) The hyperprolactinemic polycystic ovary syndrome may not be a distinct entity. Fertil Steril 38(5): 549-552.
- Işik AZ, Gülekli B, Zorlu CG, Ergin T, Gökmen O, et al. (1997) Endocrinological and clinical analysis of hyperprolactinemic patients with and without ultrasonically diagnosed polycystic ovarian changes. Gynecol Obstet Invest 43(3): 183-185.
- Nachtigall LB (2017) Cabergoline for hyperprolactinemia: getting to the heart of it. Endocrine 57(1): 3-5.
- Ferrero H, Garcia-Pascual CM, Pellicer N, SimónC, Pellicer A, et al. (2015) Dopamine agonist inhibits vascular endothelial growth factor protein production and secretion in granulosa cells. Reprod Biol Endocrinol 13: 104.
- Matthews ML (2015) Abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol Clin N Am 42(1): 103-115.
- Rose BI, Brown SE (2020) A review of the physiology behind letrozole applications in infertility: are current protocols optimal? J Assist Reprod Genet 37(9): 2093-2104.
- Teede HJ, Misso ML, Costello MF, Dokras A, Laven J, et al. (2018) Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Fertil Steril 110(3): 364-379.
- Mascarenhas M, Balen AH (2020) Treatment update for anovulation and subfertility in polycystic ovary syndrome. Current Opinion in Endocrine and Metabolic Research 12: 53-58.
- Cunha A, Póvoa AM (2021) Infertility management in women with polycystic ovary syndrome: a review.
Porto Biomed J 6(1): - Ajossa S, Paoletti AM, Guerriero S, Floris S, Mannias M, et al. (1999) Effect of chronic administration of cabergoline on uterine perfusion in women with polycystic ovary syndrome. Fertil Steril 71(2): 314-318.
- Papaleo E, Doldi N, Santis DL, Marelli G, Marsiglio E, et al. (2001) Cabergoline influences ovarian stimulation in hyperprolactinemic patients with polycystic ovary syndrome. Hum Reprod 16(11): 2263-2266.
- Paoletti AM, Cagnacci A, Depau GF, Orrù M, Ajossa S, et al. (1996) The chronic administration of cabergoline normalizes androgen secretion and improves menstrual cyclicity in women with polycystic ovary syndrome. Fertil Steril 66(4): 527-532.
- Gómez R, Ferrero H, Delgado-Rosas F, Maria G, Concepción M, et al. (2011) Evidences for the existence of a low dopaminergic tone in polycystic ovarian syndrome: implications for OHSS development and treatment. J Clin Endocrinol Metab 96(8): 2484-2492.
- Elsersy MA (2017) Efficacy of Combined Cabergoline and Metformin Compared to Metformin Alone on Cycle Regularity in Patients with Polycystic Ovarian Disease with Hyperprolactinemia: A Randomized Clinical Trial. The Journal of Obstetrics and Gynecology of India. (September–October 2017) 67(5): 363-369.
- Zahran KM, Mostafa WA, Abbas AM, Khalifa MA, Sayed GH, et al. (2018) Clomiphene citrate plus cabergoline versus clomiphene citrate for induction of ovulation in infertile euprolactinemic patients with polycystic ovary syndrome: A randomized clinical trial. Middle East Fertility Society Journal 23(3): 173-177.
- Prelevic GM, Wurzburger MI, Peric LJA (1987) Acute effects of L-dopa and bromocriptine on serum PRL, LH and FSH levels in patients with hyperprolactinemic and normoprolactinemic polycystic ovary syndrome. J Endocrinol Invest 10(4): 389-395.
- Ghaneei A, Jowkar A, Hasani Ghavam MR, Ghaneei ME, et al. (2015) Cabergoline plus metformin therapy effects on menstrual irregularity and androgen system in polycystic ovary syndrome women with hyperprolactinemia. Iran J Reprod Med 13(2): 93-100.
- Chen H, Fu J, Huang W (2016) Dopamine agonists for preventing future miscarriage in women with idiopathic hyperprolactinemia and recurrent miscarriage history. Cochrane Database Syst Rev 7(7): CD008883.






























